| |
CE: Viral Hepatitis: New U.S. Screening Recommendations, Assessment Tools, and Treatments ".....focusing on the role of nurses in prevention and treatment"
|
| |
| |
Download the PDF here

http://journals.lww.com/ajnonline/pages/articleviewer.aspx?year=2015&issue=07000&article=00023&type=Fulltext
http://journals.lww.com/ajnonline/Fulltext/2015/07000/2_5_CE_Test_Hours___Viral_Hepatitis___New_U_S_.24.aspx
AJN, American Journal of Nursing: July 2015
Dan, Corinna MPH, RN; Moses-Eisenstein, Michelle MPH; Valdiserri, Ronald O. MD, MPH
Corinna Dan is viral hepatitis policy advisor, Michelle Moses-Eisenstein is a public health analyst, and Ronald O. Valdiserri is director, all in the Office of HIV/AIDS and Infectious Disease Policy, U.S. Department of Health and Human Services, Washington, DC. Contact author: Corinna Dan, corinna.dan@hhs.gov. The authors and planners have disclosed no potential conflicts of interest, financial or otherwise.
Abstract
Overview: Over the past 15 years, the incidences of hepatitis A and B virus infection in the United States have declined significantly. By contrast, the incidence of hepatitis C virus infection, formerly stable or in decline, has increased by 75% since 2010. Suboptimal therapies of the past, insufficient provider awareness, and low screening rates have hampered efforts to improve diagnosis, management, and treatment of viral hepatitis. New screening recommendations, innovations in assessment and treatment, and an updated action plan from the U.S. Department of Health and Human Services (HHS) seem likely to lead to significant progress in the coming years. This article reviews the epidemiology, natural history, and diagnosis of viral hepatitis; discusses new screening recommendations, assessment tools, and treatments; and outlines the HHS action plan, focusing on the role of nurses in prevention and treatment.
In the United States, the epidemiology of viral hepatitis, most commonly caused by hepatitis A, B, or C viruses, has changed dramatically over the past three decades. With the development of vaccines for hepatitis A and B viruses and the introduction of universal precautions and blood supply safety measures, hepatitis A virus (HAV) and hepatitis B virus (HBV) infection rates have declined by 88% and 64%, respectively, since 2000.1 By contrast, after years of stable or declining rates, the incidence of hepatitis C virus (HCV) infection has increased by 75% since 2010.1 In 2011, the U.S. Department of Health and Human Services (HHS) released the first national, comprehensive, multiagency action plan for preventing and treating viral hepatitis.2 The HHS action plan, which was updated in 2014, outlines measures that can be taken to improve health outcomes for people infected with HBV and HCV, both of which can progress to chronic disease that often produces minimal symptoms before resulting in cirrhosis or hepatocellular carcinoma.3 With new recommendations calling for wider screening, the approval of new therapies that can cure more than 90% of those with chronic HCV, and the introduction of noninvasive technology for evaluating liver fibrosis, nurses are positioned to play a critical role in this plan-providing the clinical leadership and patient counseling that can
* reduce the number of new viral hepatitis infections.
* ensure that existing infections are diagnosed.
* minimize the impact of chronic infection on the patients they care for and the communities they serve.
This article reviews the epidemiology and diagnosis of HAV, HBV, and HCV; discusses the natural history of chronic HBV and HCV; and outlines the HHS action plan, focusing on the role of nurses in prevention, treatment, and-in the case of HCV-cure.
SYMPTOMS, EPIDEMIOLOGY, AND PREVENTION
In the acute phase of infection, HAV, HBV, and HCV may produce similar symptoms (see Common Signs and Symptoms of Acute Viral Hepatitis A, B, and C). The viruses, however, differ from each other in terms of mode of transmission, natural history, and vaccine availability (see Table 1 1, 4, 5).
HAV is spread primarily through the fecal-oral route, either from person to person, or through exposure to contaminated food or water.4 Typically, the virus causes a self-limited infection of less than two months' duration, which confers immunity when resolved.4 Most acute infections (85%) are symptomatic.6 In rare cases, acute HAV infection can result in death. In 2012, the Centers for Disease Control and Prevention (CDC) received 1,562 case reports of HAV-a number estimated to represent as many as 3,050 actual cases, since, as with HBV and HCV, some infected patients are asymptomatic and some fail to seek care and testing.1, 6 For HAV, each reported case is believed to represent nearly two actual infections.6 In primate studies, HAV has remained infectious on environmental surfaces for more than a month.7 Preventive measures include vaccination, proper hand hygiene, and proper food handling, as well as disinfection of contaminated surfaces.
A safe and effective vaccine for HAV has been available in the United States since 1995. In 1999, the Advisory Committee on Immunization Practices called for the routine vaccination of children living in 17 states with consistently high HAV incidence rates.8, 9 By 2003, those states saw an 88% drop in HAV incidence-to 2.5 cases per 100,000 population from the average rate of 21.1 cases per 100,000 population recorded in the prevaccination period.10 The series of two HAV vaccinations administered over a six-month period was shown to protect adults for at least 10 years and 99% of children for five to six years.10
Children, who are generally asymptomatic, have played an important role in transmitting HAV. Although initial recommendations for HAV vaccination among children focused on states with high incidence rates, the recommendation has since been expanded to include all infants, starting at one year of age, as part of the primary childhood immunization series.10 Unvaccinated adults should be considered for HAV vaccination if they are at risk for exposure or severe adverse consequences of infection (see HAV Vaccination Recommendations 10).
HBV is transmitted by percutaneous or mucosal exposure to the body fluids of an infected person.11 In the United States, the most common modes of HBV transmission are sexual contact and injection drug use.1, 4 Perinatal transmission is estimated to have occurred in 1,058 of all U.S. births in 2009.12
In 2012, the CDC received reports of 2,895 cases of acute HBV infection, suggesting that the actual number of new cases in the United States (including asymptomatic patients and those who seek no care) was actually closer to 19,000,1 as each reported case of HBV is estimated to represent roughly 6.5 actual infections.6 Acute infection is symptomatic in up to 50% of adult cases; infants and children are usually asymptomatic.4 Acute HBV infection most often resolves completely and confers lifelong immunity, but in up to 2% of cases, infection results in fulminant hepatitis, which has a case fatality rate of 63% to 93%.4
Acute HBV progresses to chronic infection in about 90% of infants infected at birth, 30% to 50% of children ages one to five, and 5% of adults.1 People with a compromised immune system are at elevated risk of developing chronic infection. Those with chronic infection tend to be asymptomatic carriers, though roughly 25% eventually develop cirrhosis or hepatocellular carcinoma. An estimated 240 million people worldwide and 700,000 to 1.4 million people in the United States are chronically infected with HBV.1, 13
HBV is considered hardy and remains infectious outside the body for about seven days.4 To prevent HBV infection, people are advised to get vaccinated, avoid unprotected sexual contact, and avoid percutaneous or mucosal contact with infected blood, body fluids, and items that have been in contact with potentially infected blood or body fluids, such as needles and other equipment used to inject drugs.
A safe and effective vaccine for HBV has been available since 1986.4 It was first used to protect adults at risk, but a comprehensive infant and childhood vaccination strategy was introduced in 1991 (see HBV Vaccination Recommendations 14-17).14 Between 2000 and 2012, the rate of new HBV infections in the United States declined by 64%, primarily because of this effective immunization strategy.1 In adults, the series of three HBV vaccinations should be administered within a six-month period regardless of age, with no need to restart the series if the intervals between injections exceed recommendations.4 (CDC vaccine recommendations for infants, children, and adolescents can be found at www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/hepb.html.) Three doses of the vaccine provide protection for 98% to 100% of vaccinated infants and 90% to 95% of vaccinated adolescents and adults.4
HCV is transmitted parenterally, usually through injection drug use and less frequently through poor infection control practices during health care procedures.18 Sexual transmission occurs rarely, though among both men who have sex with men and heterosexuals, the likelihood of sexual transmission rises with the number of sex partners and when sex partners are coinfected with HIV.18-20 In addition, there is evidence that men who have sex with men and take HIV preexposure prophylaxis may be at risk for sexual transmission of HCV if they have unprotected sex with HCV-infected partners.21
In 2012, the CDC received reports of 1,778 cases of acute HCV infection,1 which suggests that the actual number of acute cases was 21,869, since each reported case is estimated to represent 12.3 actual cases.6 The CDC also documented a 75% increase in new HCV infections between 2010 and 2012 and linked the increase to rising injection drug use, primarily among white adolescents and young adults who reside in nonurban settings.1
Acute infection progresses to chronic disease in 75% to 85% of cases.1 Infection is usually asymptomatic, even in the presence of mild to severe liver disease.1 Chronic HCV infection is the leading cause of liver-related death and hepatocellular carcinoma in the Western world, with persistent inflammation causing cirrhosis within 30 years in 10% to 20% of those infected and putting 1% to 5% of these patients at annual risk for hepatocellular carcinoma.5
As many as 150 million people worldwide and up to 3.9 million people in the United States are chronically infected with HCV.1, 13 Little is known about the factors associated with spontaneous clearance of the virus, though immune response is determined in part by genetics, sex, and mode of acquisition.5
HCV is considered very hardy; a recent study found that cell culture-derived HCV can remain infectious on surfaces up to six weeks, suggesting that accidental contact with contaminated surfaces may be a source of health care-associated transmission.22 In light of these findings, health care providers would be well advised to avoid percutaneous exposure to infected blood. Currently there is no vaccine for HCV.

SEROLOGIC TESTING FOR VIRAL HEPATITIS
To confirm a specific diagnosis of HAV, HBV, or HCV, serologic testing is required (see Table 2 1, 16, 18, 36). Screening tests are generally conducted on asymptomatic people with an increased probability of having or developing a disease, whereas diagnostic testing is conducted to establish the presence of a disease, typically in patients who are symptomatic. Previous screening strategies for HBV and HCV were based on the identification of such potentially stigmatizing risk factors as a history of injection drug use or unprotected sex with multiple partners, which were seldom assessed by providers or disclosed by patients. Because this strategy failed to identify many patients who were chronically infected with HBV or HCV, the CDC and the U.S. Preventive Services Task Force (USPSTF) now recommend expanded HBV and HCV serologic screening for all people considered to be at high risk as well as for members of groups known to have high prevalence rates, such as people born between 1945 and 1965 and those born in certain countries or regions.11, 23-25 Both HBV and HCV serologic screening of patients at high risk for infection are Grade B USPSTF recommendations, meaning that they qualify as preventive services covered without copay under the Affordable Care Act (ACA).
HBV serologic screening is recommended for the following groups11, 23:
* people born in countries or regions with a chronic HBV prevalence of 2% or more, or born in the United States but not vaccinated at birth
* people living with HIV
* people who use injection drugs
* men who have sex with men
* household members or sexual partners of someone with HBV infection
* patients who have weakened immune systems or are undergoing hemodialysis
HCV serologic screening. Those with the following risk factors should undergo HCV serologic screening at least once24, 26:
* birth date between 1945 and 1965, regardless of other risk factors
* receipt of clotting factor concentrates produced before 1987
* persistently abnormal alanine aminotransferase levels
* history of long-term hemodialysis treatment
* receipt of blood, blood products, or an organ transplant prior to July 1992 or receipt of blood from a donor who later tested positive for HCV
Those in the following groups should undergo HCV serologic screening routinely24, 26:
* people with HIV infection
* anyone who has ever used injection drugs (even once)
* health care, emergency medical, and public safety workers after needlestick injury or mucosal exposure to HCV-contaminated blood
* children born to mothers who test positive for HCV
HCV screening is a two-step process. People recommended for screening should first be tested using an HCV antibody test. A positive antibody test indicates a history of exposure to HCV but does not distinguish resolved from active infections. Since HCV antibodies can persist for years following a naturally resolved infection or successful antiviral therapy, further testing with a nucleic acid test is required to determine whether infection is active.

EMERGING OPPORTUNITIES TO ADDRESS HEPATITIS C
Since more than 1% of the U.S. population is estimated to be chronically infected with HCV,1 nurses are likely to see patients with chronic HCV infection in their practices. The recent rise in new HCV infections among young people further underscores the need to screen patients. Past efforts to address HCV have been hampered by therapies with limited efficacy, a lack of awareness among providers, and low screening rates. Today, however, recently approved antiviral therapies have fewer adverse effects, with some having cure rates of 90% to 100% in clinical trials.27 Furthermore, the ACA has expanded access to hepatitis screening while encouraging previously uninsured people to enter the health care system. All of this creates important opportunities for nurses to improve health outcomes for patients with viral hepatitis, particularly those with HCV.
Potential HCV manifestations. Since most patients with chronic HCV infection are asymptomatic until they develop advanced liver disease, it's important to maintain a high level of clinical suspicion. Mild to moderate liver damage may produce vague symptoms, such as fatigue, over a period ranging from years to decades. HCV, however, may also have a number of extrahepatic manifestations because it's associated with cryoglobulinemia and a lymphoproliferative immune complex disorder that causes arthralgia, purpura, glomerular disease, peripheral neuropathy, central nervous system vasculitis, and reduced complement levels.28
The CDC estimates that29
* of those who are chronically infected with HCV, only half have been diagnosed.
* fewer than 38% of those with an HCV diagnosis have been referred to care.
* fewer than 11% of those referred to care have been treated.
Identifying extrahepatic manifestations of chronic HCV infection may increase the likelihood of diagnosis, referral, and treatment (and now the possibility of cure). As we work to understand the true burden of HCV in the United States and how to better target the use of limited resources, accurate diagnosis and appropriate reporting are paramount.

EVALUATING LIVER DISEASE
All patients who have both a positive HCV antibody test and a positive nucleic acid test are considered to have ongoing infection. They should be evaluated for liver disease and receive counseling and disease management services. Liver disease evaluation involves determining liver function and the degree of liver damage or fibrosis. Severity of fibrosis is usually described in terms of a Metavir score, which ranges from F0, indicating no fibrosis, to F4, indicating cirrhosis. Historically, liver disease was evaluated on the basis of a liver biopsy, which was invasive, expensive, not without discomfort, and susceptible to sampling error, meaning that the findings could vary depending on the portion of the liver sampled. In 2013, however, the U.S. Food and Drug Administration approved a noninvasive technology known as transient elastography (FibroScan) for determining the degree of liver fibrosis. The technology's accuracy is improved when it is paired with serologic tests developed to monitor liver disease progression. Following liver evaluation, providers should discuss possible treatment options with patients and teach them how to maintain optimal liver health, even if antiviral treatment is not prescribed at that time.
HCV COUNSELING, EDUCATION, AND INTERVENTION
All patients with chronic HCV infection should receive education, counseling, and intervention to reduce the likelihood of liver disease progression and viral transmission to others. The CDC recommends that when providers share positive HCV test results with patients, they also evaluate patients' level of alcohol use.26 Screening adults ages 18 and older for alcohol misuse and providing brief behavioral counseling to reduce alcohol misuse is a Grade B recommendation of the USPSTF.30 As with HCV and HBV screening, screening for alcohol misuse is covered as a preventive service without copay under the ACA.
For people with substance use disorders and those at risk for developing such disorders, the Substance Abuse and Mental Health Services Administration recommends screening, brief intervention, and referral to treatment (SBIRT).31
The SBIRT process provides a standardized framework that enables nurses to
* quickly assess the severity of substance use and the appropriate level of treatment.
* focus on increasing the patient's insight and awareness regarding substance use.
* motivate the patient to change risky behavior.
* identify patients in need of specialty care and help them gain access.
To protect the liver from further harm, counsel patients to
* reduce or discontinue alcohol consumption.
* avoid taking new medications, including over-the-counter and herbal agents, without first checking with their health care provider.
* obtain HIV risk assessment and testing.
* receive HAV and HBV vaccination (if not immune).
To minimize the risk of transmission to others, advise patients not to
* donate blood, tissue, or semen.
* share appliances that might come into contact with blood, such as toothbrushes, dental appliances, razors, or nail clippers.
* share needles or other injection equipment if using injection drugs.
Encourage overweight or obese patients to lose weight by following a healthy diet and staying physically active. Remind them that excess weight can cause or exacerbate liver damage.

HCV TREATMENT
The goal of HCV therapy is to achieve sustained viral response (SVR), or viral clearance, which represents a cure for HCV.32 HCV treatments have been shown to be effective and to reduce the sequelae of untreated chronic infection. Treatments, however, are underutilized for a number of reasons. Notably, most patients are unaware of their HCV status, patients and many providers are unaware of new treatments, and the policies of insurance companies and other payers often restrict access to treatment (by requiring evidence of advanced liver fibrosis or cirrhosis before approving treatment, for example).
In addition, older HCV therapies were very difficult to tolerate, with many adverse effects and numerous contraindications. Since 2001-and until very recently-the standard of care for HCV has been pegylated interferon (peginterferon) injected weekly and paired with oral ribavirin. Both patients and providers have been anxious about initiating the 48-week therapy regimen because of its considerable adverse effect profile and relatively low rate of SVR. In two large phase 3 clinical trials of peginterferon plus ribavirin, at least 20% of participants reported adverse events, with 43% to 64% of these reporting fatigue, headache, and fever; 28% to 43% reporting nausea, alopecia, and insomnia; and 22% to 31% reporting depression.33
In the United States, peginterferon plus ribavirin is no longer the treatment of choice for most genotypes of HCV, though it is listed in the current guidelines28 as an alternative therapy for genotype 5 and is still used in some countries that have not yet approved the newest antiviral agents. The approval of new combination antiviral treatments-such as simeprevir (Olysio) plus sofosbuvir (Sovaldi), ledipasvir-sofosbuvir (Harvoni), and ombitasvir-paritaprevir-ritonavir packaged with dasabuvir (Viekira Pak)-which have fewer adverse effects, shorter treatment durations (12 to 24 weeks), and higher cure rates, has changed the HCV treatment landscape. Additional HCV treatments are in development and are expected to become simpler and even more effective in curing chronic HCV. The availability of effective, curative treatments should serve as a great incentive to diagnose HCV early.
NATIONAL VIRAL HEPATITIS ACTION PLAN
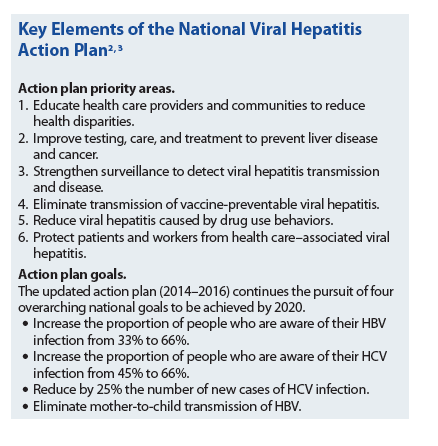
In 2010, the Institute of Medicine (IOM) released a report that identified viral hepatitis as an underappreciated public health problem.2 The report highlighted multiple barriers to viral hepatitis prevention and control and provided specific recommendations to improve efforts to stop the spread of viral hepatitis. In response to the IOM report, HHS developed and released Combating the Silent Epidemic of Viral Hepatitis: Action Plan for the Prevention, Care and Treatment of Viral Hepatitis in May 2011.2 On April 3, 2014, an updated version of the action plan was released for the years 2014 through 2016.3 Detailing over 150 specific actions for federal agencies to take by 2016, the action plan sets goals within six major priority areas and provides a framework to focus the efforts of federal and nonfederal stakeholders in monitoring and measuring the achievement of these goals (see Key Elements of the National Viral Hepatitis Action Plan 2, 3).
The major federal contributors to the action plan (HHS, the Department of Justice's Federal Bureau of Prisons, the Department of Housing and Urban Development, and the Department of Veterans Affairs) recognize that its goals cannot be fully achieved without significant participation from other, nonfederal partners. As such, the action plan seeks to engage a broad range of stakeholders in efforts to address viral hepatitis in the United States. Because of the pivotal role that nurses play as educators, care coordinators, and health care providers, they are uniquely positioned to provide critical leadership in advancing our nation's efforts to address the "silent epidemic" of viral hepatitis.
THE CRITICAL ROLE OF NURSES
Nurses play an important role in all six priority areas of the action plan. As educators, nurses can give accurate information to patients and, as members of the health care team, help improve the quality of HCV care by ensuring that recommended screening, HAV and HBV vaccination, and HCV treatment are provided. For a variety of online educational resources, see Resources for Nurses and Their Patients.
In 2012, the American Academy of Nursing published a policy statement calling for a national response to inadequate HCV screening and testing. The statement concludes as follows: "Leveraging local, state and federal resources as well as the expertise of advanced practice nurses and RNs can provide accurate education [and] information regarding screening, testing, surveillance and evaluation of [the] impact of strategies. Nurses, and their vast health promotion networks, are poised to best assess, intervene and evaluate the impact of enforcing these recommendations."34
In practice settings, nurses may be responsible for
* reporting communicable diseases to state and local health departments, thereby improving surveillance data in their communities.
* assisting in disease outbreak investigations, helping to identify sources of infection, and protecting people from exposure.
* educating patients on recommended vaccines, including those for HAV and HBV, as research has shown that patients are more likely to accept a service if recommended by a health care provider.35
* educating parents on infant HBV, ensuring that standing orders for HBV vaccination are in place, and vaccinating all infants in their care within 12 hours of birth.
* counseling patients with chronic HBV or HCV infection about liver health and treatment.
* assessing patients for alcohol or substance use, making referrals as appropriate.
Nurses can also help to identify patients who are using substances at initial assessments and during annual physical examinations, or when patients present with an accidental injury or wound that may be related to injecting drugs. Upon identifying a patient at risk, they can
* refer patients to substance abuse treatment programs.
* confirm HBV vaccination completion and HCV screening.
* provide education on HCV transmission.
Nurses also play a significant role in infection control. They can identify and address threats to sterile technique before such threats put patients or other health care workers at risk for exposure to such blood-borne pathogens as HBV and HCV. By ensuring that HBV vaccination coverage for all health care workers is in accordance with the Occupational Safety and Health Administration guidelines, nurses can help protect their colleagues from HBV infection due to accidental needlestick. As leaders in preventing new hepatitis infections, providing care and treatment for those living with viral hepatitis, and ensuring the safety of patients and providers in health care settings, nurses are integral to achieving the goals of the HHS viral hepatitis action plan.

|
|
| |
| |
|
|
|