| |
Abbvie GT1b GIFT-1/TURQUOISE-III
|
| |
| |
Download the PDF here
(TURQUOISE-III) - AbbVie Announces New Phase 3b Results in Genotype 1b Chronic Hepatitis C Patients with Compensated Liver Cirrhosis - 100% SVR - (06/24/15)
TURQUOISE-III: SAFETY AND EFFICACY OF 12-WEEK RIBAVIRIN-FREE TREATMENT FOR PATIENTS WITH HCV GENOTYPE 1B AND CIRRHOSIS - (06/26/15) .....15th International Symposium on Viral Hepatitis and Liver Disease, Berlin, Germany, 26 June 2015
EASL: OMBITASVIR/PARITAPREVIR/RITONAVIR FOR TREATMENT OF HCV GENOTYPE 1B IN JAPANESE PATIENTS WITH OR WITHOUT CIRRHOSIS: RESULTS FROM GIFT-I - (04/28/15)
AbbVie's Investigational Chronic Hepatitis C Treatment Granted Priority Review in Japan......http://abbvie.mediaroom.com/2015-04-15-AbbVies-Investigational-Chronic-Hepatitis-C-Treatment-Granted-Priority-Review-in-Japan
----------------------
Randomized Phase 3 Trial of Ombitasvir/Paritaprevir/Ritonavir for HCV Genotype 1b-infected Japanese Patients With or Without Cirrhosis
Hepatology July 3 2015
Hiromitsu Kumada,1* Kazuaki Chayama,2 Lino Rodrigues-Jr,3 Fumitaka Suzuki,1 Kenji Ikeda,1
Hidenori Toyoda,4 Ken Sato,5 Yoshiyasu Karino,6 Yasushi Matsuzaki,7 Kiyohide Kioka,8 Carolyn
Setze,3 Tami Pilot-Matias,3 Meenal Patwardhan,3 Regis A Vilchez,3 Margaret Burroughs,3
Rebecca Redman3
ABSTRACT
GIFT-I is a phase 3 trial evaluating efficacy and safety of a 12-week regimen of co-formulated ombitasvir(OBV)/paritaprevir(PTV)/ritonavir(r) for treatment of Japanese HCV genotype(GT) 1b-infected patients. GIFT-I consists of a double-blind, placebo-controlled substudy of patients without cirrhosis and an open-label substudy of patients with compensated cirrhosis. Patients without cirrhosis were randomized 2:1 to once daily OBV/PTV/r(25mg/150mg/100mg; Group A) or placebo(Group B). Patients with cirrhosis received open-label OBV/PTV/r(Group C). The primary efficacy endpoint was the rate of sustained virologic response 12 weeks post-treatment(SVR12) in interferon-eligible, treatment-naïve patients without cirrhosis and HCV RNA ≥100,000 IU/ml in Group A.
ombitasvir(OBV, an NS5A inhibitor) and paritaprevir(PTV, a NS3/4A protease inhibitor
A total of 321 patients without cirrhosis were randomized and dosed with double-blind study drug(106 received double-blind placebo and later received open-label OBV/PTV/r) and 42 patients with cirrhosis were enrolled and dosed with open-label OBV/PTV/r.
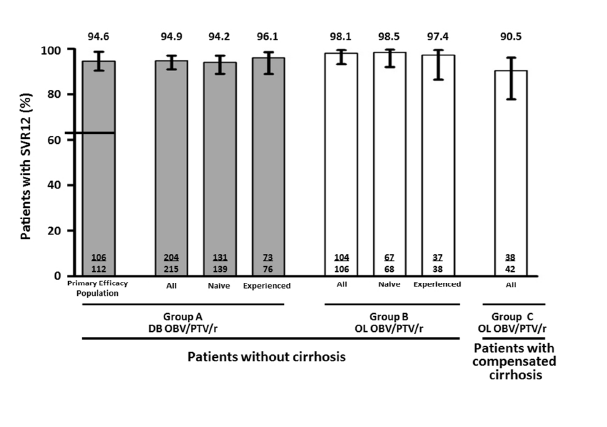
In the primary efficacy population, the SVR12 rate was 94.6%(106/112; 95% confidence interval 90.5-98.8). SVR12 rates were 94.9%(204/215) in Group A, 98.1%(104/106) in Group B(open-label), and 90.5%(38/42) in Group C.
Overall, virologic failure occurred in 3.0%(11/363) of patients who received OBV/PTV/r. The rate of discontinuation due to adverse events was 0-2.4% in the three patient groups receiving OBV/PTV/r. The most frequent adverse event in patients in any group was nasopharyngitis.
Conclusion: In this broad HCV GT1b-infected Japanese patient population with or without cirrhosis, treatment with OBV/PTV/r for 12 weeks was highly effective and demonstrated a favorable safety profile.
In Japan, it is estimated that 2 million people are infected with hepatitis C virus(HCV)(1). Prevalence of HCV infection increases with age in the Japanese population(2). While HCV genotype(GT) 1a infection is common in North America and Western Europe, in Japan approximately 99% of HCV GT1-infected patients have GT1b(3).
RESULTS
Baseline Patient Demographics and Characteristics
Of 467 patients screened, 321 patients without cirrhosis were randomized in Substudy 1(215 to double-blind OBV/PTV/r[Group A], 106 to double-blind placebo[Group B]) and 42 patients with cirrhosis were enrolled in Substudy 2(open-label OBV/PTV/r[Group C])(Figure 1 and Supplemental Figure 1). All enrolled patients received at least one dose of study drug. The baseline characteristics of patients without cirrhosis in Groups A and B(Substudy 1) were generally similar(Table 1). Among patients with cirrhosis(Substudy 2), 78.6% were treatment-experienced, and mean(standard deviation) baseline platelet count, albumin, and international normalized ratio(PT-INR) were 114.2(47.4)x109 cells/L, 38.2(3.9) g/L, and 1.060(0.091), respectively.
Virologic Response
Rapid virologic and end of treatment responses and SVR4 for the three patient groups are presented in Supplemental Table 2. SVR12 rates for patients with and without cirrhosis receiving OBV/PTV/r are displayed in Figure 2. In the primary efficacy population, the SVR12 rate was 94.6%(106/112, 95% CI 90.5-98.8). The lower bound of the 95% CI was 90.5%, establishing superiority of the OBV/PTV/r regimen to the predefined threshold(Figure 2). The overall SVR12 rate among patients without cirrhosis in Group A was 94.9%(204/215); the SVR12 rates in all treatment-naïve and treatment-experienced patients were 94.2%(131/139) and 96.1%(73/76) respectively.
The overall SVR12 rate in patients without cirrhosis receiving open-label OBV/PTV/r(Group B) was 98.1%(104/106); SVR12 rates in treatment-naïve and treatment-experienced patients were 98.5%(67/68) and 97.4%(37/38) respectively in this group. The overall SVR12 rate in patients with cirrhosis receiving open-label OBV/PTV/r(Group C) was 90.5%(38/42), including 100%(9/9) and 87.9%(29/33) in treatment-naïve and treatment-experienced patients, respectively. SVR12 rates for all other predefined subpopulations were greater than 90%(Table 2).
ALT Normalization
In patients without cirrhosis with ALT levels>ULN at baseline, ALT normalized at the end of the double-blind treatment period in a significantly greater proportion in patients receiving OBV/PTV/r versus placebo(94.3%[116/123] versus 18.9%[10/53]; P<0.001).
Virologic Failure
Virologic failure(OTVF or relapse) occurred in 3.0%(11/363) of patients who received OBV/PTV/r(double-blind and open-label periods). Virologic failure occurred in 2.8%(6/215), 1.9%(2/106), and 7.1%(3/42) of patients in Groups A, B, and C, respectively. OTVF occurred in 0.5%(1/215), 0.9%(1/106), and 2.4%(1/42) of patients in Groups A, B, and C, respectively. Relapse occurred in 2.4%(5/209), 1.0%(1/105), and 5.0%(2/40) of patients in Groups A, B, and C, respectively. Additional information on patients who experienced virologic failure is in Supplemental Table 3.
Resistance Associated Variants
RAVs in NS3/4 and NS5A were detected in 1% and 38% of patients at baseline, respectively. The most commonly detected NS3A and NS5A RAVs in baseline samples were D168E(4/351, 1%) and Y93H(49/357, 14%), respectively. RAVs were observed in both NS3 and NS5A at the time of virologic failure in 10 of the 11 patients who experienced OTVF or relapse. In NS3, D168V alone or in combination with Y56H was observed in 73%(8/11) of patients, D168A in combination with Y56H was observed in 2 patients, and 1 patient did not have any treatment emergent RAVs in NS3. In NS5A, Y93H was pre-existing in 8 patients and at the time of failure; Y93H alone or in combination with L28M, R30Q, L31M, L31V, and/or P58S was observed in 91%(10/11) of patients; L31F was observed in 1 patient.
Safety
Rates of treatment-emergent adverse events(TEAEs) in the three patient groups are in Table 3. During the double-blind period, a greater percentage of patients without cirrhosis receiving OBV/PTV/r than placebo experienced TEAEs(68.8%[148 of 215 patients] versus 56.6%[60 of 106 patients], P<0.05)(Table 3). TEAEs were predominantly Grade 1 or 2 in severity. TEAEs occurring with a frequency greater than 5% among patients without cirrhosis during the double-blind period in either treatment group were nasopharyngitis(16.7%[36 patients], OBV/PTV/r; 13.2%[14 patients], placebo), headache(8.8%[19 patients], OBV/PTVr; 9.4%[10 patients], placebo), and peripheral edema(5.1%[11 patients], OBV/PTV/r; 0%, placebo). The only TEAE significantly more frequent with OBT/PTV/r versus placebo during the double-blind period was peripheral edema. The proportions of serious TEAEs and TEAEs leading to study drug discontinuation were not significantly different in patients receiving OBV/PTV/r versus placebo(3.3%[7 patients] versus 1.9%[2 patients], P>0.05; and 0.9%[2 patients] versus 0%, P>0.05, respectively). TEAEs leading to study drug discontinuation in patients receiving OBV/PTV/r were anuria and hypotension in one patient each.
The TEAE profile in patients without cirrhosis receiving open-label OBV/PTV/r was comparable to that of patients without cirrhosis receiving double-blind OBV/PTV/r(Table 3). TEAEs were predominantly Grade 1 or 2. TEAEs occurring with a frequency greater than 5% in this group were nasopharyngitis(7.5%[8 patients]) and headache(6.6%[7 patients]). Peripheral edema occurred in 3.8%(4 patients) of patients. Serious TEAEs occurred in 2.8%(3 patients) of patients in this group, and no patient discontinued treatment due to TEAEs.
Among patients with cirrhosis receiving open-label OBV/PTV/r, 73.8%(31 of 42 patients) experienced at least 1 TEAE(Table 3). TEAEs were predominantly Grade 1 or 2 in severity. TEAEs occurring with a frequency greater than 5% were nasopharyngitis(14.3%[6 patients]), pyrexia(9.5%[4 patients]), nausea(7.1%[3 patients]), peripheral edema(7.1%[3 patients]), decreased platelet count(7.1%[3 patients]), and headache(7.1%[3 patients]). Serious TEAEs occurred in 4.8%(2 patients) of patients with cirrhosis. One patient(2.4%) had a serious TEAE(pulmonary edema) that led to study drug discontinuation.
All patients in the study who experienced a TEAE of peripheral edema were using concomitant calcium channel blockers(CCBs). Additional analyses indicated that the incidence of any edema-related TEAEs(defined as peripheral edema, edema, face edema, or pulmonary edema) was related to the use and dose of CCBs(Supplemental Tables 4 and 5).
There were no deaths due to a TEAE in any patient group; however, two deaths occurred more than 30 days after the end of treatment. One patient was a 71-year-old female with compensated cirrhosis who experienced a fatal non-related, non-treatment emergent adverse event of lymphangiosis carcinomatosa on post-treatment Day 76. HCV RNA levels were< LLOQ at all measurements from open-label treatment Day 11 until post-treatment Day 54, the last timepoint measured. The other patient was a 65-year-old female with compensated cirrhosis who achieved SVR12 and experienced a non-related, non-treatment emergent adverse event of hepatocellular carcinoma on Post-treatment Day 84 and died on post-treatment day 253.
Post-baseline laboratory values of grade 3 or higher are presented in Table 3. There were no grade 4 values for any laboratory parameter. One diabetic patient without cirrhosis receiving double-blind OBV/PTV/r had an asymptomatic ALT value>5X ULN, concurrent with acute worsening of glycemia during betamethasone use. ALT peaked at treatment day 57(556 IU/mL) and resolved while continuing DAA treatment. In this patient, discontinuation of betamethasone was followed by decrease of blood glucose levels and ALT declines. This patient completed study drug and achieved SVR12. Another patient without cirrhosis receiving double-blind placebo had elevations in ALT and AST>5X ULN. There were no grade 3 or higher elevations in total bilirubin in patients without cirrhosis. One patient with cirrhosis receiving open-label OBV/PTV/r had a total bilirubin elevation>3X ULN. This patient was diagnosed with hepatocellular carcinoma on Post-treatment Day 84 and died on post-treatment day 253. No patient met biochemical criteria for Hy's law. There were no hemoglobin decreases<8 g/dL. No patient received erythropoietin or blood transfusions during the study. No patient had a decrease in platelet count below 50x109/L.
|
|
| |
| |
|
|
|