| |
Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease
|
| |
| |
Download the PDF here
Journal of Hepatology June 18 2015
Bhamidimarri Kalyan Ram, Czul Frank, Peyton Adam, Levy Cynthia, Hernandez Maria, Jeffers Lennox
University of Miami, Division of Medicine, Department of Hepatology, United States
Roth David, University of Miami, Division of Medicine, Department of, Nephrology, United States
Schiff Eugene, O'Brien Christopher, Martin Paul
University of Miami, Division of Medicine, Department of Hepatology, United States
To the Editor:
Hepatitis C (HCV) is a leading cause of chronic liver disease worldwide and its prevalence in hemodialysis patients is higher than that of general population. There are no approved direct acting antiviral treatments to date in this population. We describe our experience of open label treatment with simeprevir and half-dose sofosbuvir in 15 HCV GT1 patients with end stage renal disease (ESRD), defined as glomerular filtration rate (GFR) of ≤30 ml/min per 1.73 m2 or requirement for dialysis.
Methods
Of the 15 patients, 12 (80%) were on dialysis (11 hemodialysis, one peritoneal) and the rest had a GFR between eight and 15 ml/min. The University of Miami IRB approved the study and the patients were followed between January 2014 and January 2015. Patients were between the ages of 39 and 77 years infected with HCV genotype 1 (67% GT1a) and had a detectable viral load with a mean HCV RNA of 9.7 million IU/ml. HCV RNA was estimated by quantitative real time PCR assay using the COBAS® Ampliprep/Cobas® Taqman® HCV test v 2.0 (LLOQ 15 IU/ml). Genotype 1a patients were not screened for resistance mutation as routine testing for the mutation is not recommended as per AASLD or EASL guidelines [[1], [2]]. Nine (60%) patients were cirrhotic, three (20%) had stage 2-3 fibrosis and three (20%) stage 0-1 fibrosis (Metavir), all documented by liver biopsy. From the cirrhotic patients, eight (89%) patients were Child-Pugh A (compensated) and one (11%) categorized as Child-Pugh B (decompensated). Six (40%) patients were naïve to treatment and nine (60%) were treatment experienced, all of them with PegIFN and ribavirin (six relapsers and three null responders) and four protease inhibitors (PI) failures. Baseline characteristics are summarized in Table 1.
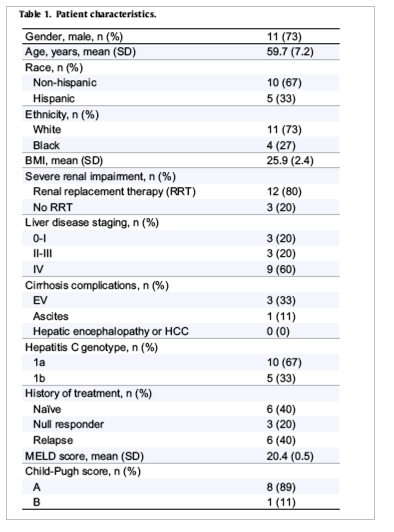
Preliminary pharmacokinetic data showed that sofosbuvir 400 mg daily in the ESRD setting is associated with high drug levels (28-171%) and extremely high GS-331007 levels (451-2070%) while 200 mg daily in non-dialysis ESRD setting resulted in minimally elevated drug levels and up to 300% of the GS-331007 levels (Table 2) [4]. Thus we hypothesized that half-dose sofosbuvir would be reasonably efficacious as seen in phase II clinical trial while mitigating potential toxicity in ESRD patients [3]. We initially used sofosbuvir 400 mg every other day but subsequently changed our strategy to sofosbuvir 200 mg daily to be administered at least one hour pre-dialysis based on data in Table 2 [[3], [4], [5], [6], [7]]. The used protocol dosing of sofosbuvir 200 mg daily was achieved by simple pill-splitting of a 400 mg tablet into two portions, with each portion ingested on consecutive days.
Results
Fourteen (93%) patients were treated for a total of 12 weeks and one patient had treatment extended to 24 weeks. In regards to the dosage, 11 (73%) patients received sofosbuvir 200 mg daily and the rest (27%) were on 400 mg every other day. All patients received the standard dose of simeprevir of 150 mg daily. No dose adjustments were performed during the length of treatment. None of the patients were on amiodarone.
Twelve (80%) patients became aviremic by week four and all patients were aviremic by week eight and remained so until the end of treatment. Sustained virologic responses (SVR) at week four and 12 were documented to be 93% and 87% respectively. Following completion of treatment one patient with HCV GT1a and other with GT1b relapsed but they both shared similar characteristics of being viremic at week four of treatment, compensated cirrhosis, hemodialysis dependent, prior PI failures with mean platelet count of 90,000/μl, mean albumin >3.7 g/dl and a mean baseline HCV RNA of 11 million IU/ml. Due to insurance approval, treatment in only one cirrhotic patient (HCV GT1a) could be extended to 24 weeks but he relapsed despite prolonged treatment.
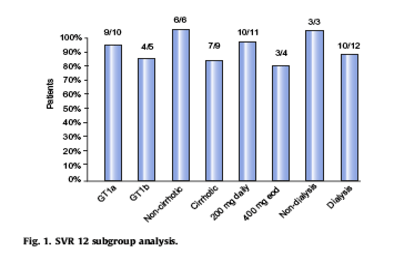
Thereafter a subgroup analysis on SVR12 was performed. For genotype GT1a vs. GT1b the SVR12 was 90% and 80% respectively, for non-cirrhotics versus cirrhotics was 100% vs. 78%, comparing dosage 200 mg daily versus 400 mg every other day was 91% vs. 75% and dialysis versus non-dialysis group was 83% vs. 100% respectively (Fig. 1). No major adverse events (AE's) were reported during treatment. Minor AE's included fatigue (20%), rash/itching (13%), anemia (13%), diarrhea and loss of appetite (7%). No patient had to interrupt therapy due to side effects. The safety of sofosbuvir-containing regimens in patients with decompensated liver disease and significantly impaired kidney function remains unclear.
Discussion
Currently, there is minimal clinical experience with the use of newly available nucleotide analog, sofosbuvir in severe renal impairment (GFR ≤30 or dialysis). Gane et al. described the safety, efficacy and pharmacokinetics of sofosbuvir plus ribavirin in ten HCV infected patients with severe renal impairment in 2014 which resulted in 40% SVR12 [4]. Experience with sofosbuvir plus simeprevir in this setting is limited to a single case report published recently by Perumpail et al., describing the successful use of similar regimen in a post-liver transplant patient who developed ESRD due to HCV associated glomerulonephritis [8].
We presented our successful experience of interferon and ribavirin-free regimen in the ESRD cohort at the International Liver Congress 2015 in Vienna [11]. As compared to other prior regimens [9], our regimen is free of both interferon and ribavirin resulting no SAE's. Two patients who developed anemia, were non-cirrhotics on dialysis, had baseline hemoglobin above 11 g/dl and both were managed conservatively without the need for blood products. It is interesting to note that SVR12 in non-cirrhotics was 100%. We acknowledge our study limitations mainly being an open label treatment experience containing a small sample size but in the given clinical realm of growing need to offer treatment in such "difficult-to-treat" patient cohort, we think our case series offers proof of concept and feasibility data for future studies. We also acknowledge that the optimal dosing of sofosbuvir in this setting needs to be formally studied with careful measurements of drug and metabolite levels.
Real world observational data (HCV TARGET) using sofosbuvir 400 mg daily in combination with other agents demonstrated an overall SVR12 of 88% but higher rates adverse events [12]. However, another case series by Nazario et al., using full dose sofosbuvir and simeprevir reported mild AE's and 100% SVR12, although only 11/17 patients completed 12 week follow-up post-treatment [13]. The safety of sofosbuvir in ESRD and decompensated cirrhosis is unclear and larger trials are awaited. ritonavir/paritaprevir, ombitasvir, dasabuvir do not need renal dose adjustment and preliminary results in RUBY-1 trial demonstrated no SAE's except for anemia when ribavirin was included in the regimen. Although full data is not yet available, this approved regimen showed excellent on-treatment response and 100% SVR4 in the ten out of 20 patients that reached post-treatment week 4 [14].
Elbasvir plus grazoprevir have antiviral activity against HCV genotypes 1 and 4, which when administered once daily in the HCV infected ESRD patients, achieved 99% SVR12 in the C-SURFER trial [15]. Elbasvir, grazoprevir and daclatasvir are yet to be approved. These futures DAA's also do not require renal dose adjustment and thus provide promising options for this cohort.
Coalescing our experience with the KDIGO recommendations we suggest that patients with chronic HCV and ESRD should be evaluated for hepatitis C treatment. One potential disadvantage of curing HCV in pre-kidney transplant patients is their ineligibility to receive an organ from HCV positive donors, which could eventually lead to prolonged time on the waiting list. However, HCV treatment in ESRD setting could be beneficial in certain scenarios 1) transplant-ineligible patients, where HCV cure can significantly reduce all-cause mortality 2) those who would undergo living donor kidney transplants, where curing HCV would lead to better graft and patient outcomes [10] 3) those with advanced fibrosis (F3-F4) but well compensated liver disease who are listed for simultaneous liver kidney transplantation (SLKT), where HCV cure could potentially avoid the need for liver transplantation in a majority of such patients 4) those who do not consent for HCV positive organs. Therefore, patients on the transplant waiting list should be ideally co-managed as a team by their transplant nephrologists and hepatologists in order to determine the timing of HCV treatment (pre or post transplant) on an individual basis.
In summary, HCV in severe renal impairment is considered a difficult-to-treat group and in whom SVR is associated with improved pre- and post-kidney transplant outcomes. Half-dose sofosbuvir plus full dose simeprevir regimen is free of interferon and ribavirin and thus is an attractive option to treat HCV GT1 in severe renal impairment/dialysis patients. The regimen appears to be safe, well-tolerated and efficacious resulting in high rates of sustained virologic response. The optimal dose of sofosbuvir in severe CKD needs to be clarified by future studies and in the absence of any approved HCV treatment for this group, our data provides information the clinicians who currently need to treat such patients with the approved DAA's.
|
|
| |
| |
|
|
|