| |
FDA Announcement Daclatasvir GT3
|
| |
| |
Download the PDF here
On July 24, 2015, FDA approved DAKLINZA (daclatasvir) a hepatitis C virus (HCV) NS5A inhibitor indicated for use with sofosbuvir for the treatment of chronic HCV genotype 3 infection. DAKLINZA is available as a 30 mg and 60 mg tablet.
DAKLINZA is the first drug that has demonstrated safety and efficacy to treat genotype 3 HCV infections without the need for co-administration of interferon or ribavirin, two FDA-approved drugs also used to treat HCV infection.
The recommended dosage of DAKLINZA is 60 mg, taken orally, once daily in combination with sofosbuvir for 12 weeks. DAKLINZA may be taken with or without food.
The optimal duration of DAKLINZA and sofosbuvir for patients with cirrhosis has not been established.
DAKLINZA requires dosage modifications due to drug interactions. The dosage of DAKLINZA is reduced to 30 mg once daily when coadministered with strong CYP3A inhibitors using the 30 mg tablet. The dosage of DAKLINZA is increased to 90 mg once daily using an appropriate combination of tablets (three 30 mg tablets or one 60 mg and one 30 mg tablet) when coadministered with moderate CYP3A inducers.
DAKLINZA is contraindicated in combination with drugs that strongly induce CYP3A and, thus, may lead to lower exposure and loss of efficacy of DAKLINZA.
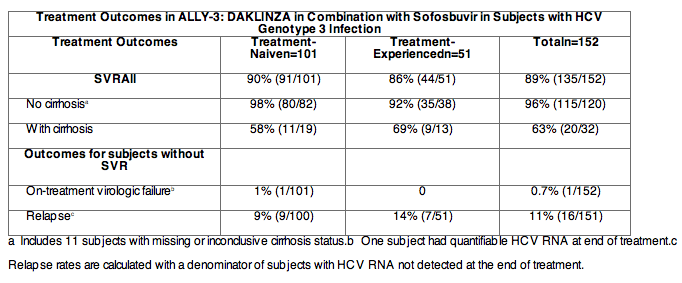
The safety and efficacy of DAKLINZA in combination with sofosbuvir were evaluated in an open-label clinical trial (ALLY-3) of 152 treatment-naïve (n=101) and treatment-experienced (n-51) participants with chronic HCV genotype 3 infection. Most treatment-experienced subjects had failed prior treatment with peginterferon/ribavirin, but 7 subjects had been treated previously with a sofosbuvir regimen and 2 subjects with a regimen containing an investigational cyclophilin inhibitor. Participants received DAKLINZA 60 mg plus sofosbuvir 400 mg once daily for 12 weeks SVR and outcomes in subjects without SVR in ALLY-3 are shown by patient population in the table below. DAKLINZA labeling carries a Limitations of Use statement to inform prescribers that sustained virologic response rates are reduced in HCV genotype 3 infected patients with cirrhosis.
Treatment Outcomes in ALLY-3: DAKLINZA in Combination with Sofosbuvir in Subjects with HCV Genotype 3 Infection
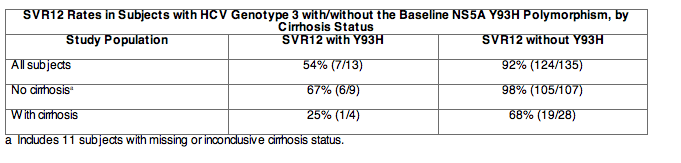
In an analysis of 148 subjects with available baseline resistance data in ALLY-3, virus from 52% (77/148) of subjects had baseline NS5A polymorphisms at resistance-associated positions (defined as any change from reference at NS5A amino acid positions 28, 30, 31, 58, 62, 92, or 93) identified by population sequencing. The Y93H polymorphism was detected in 9% (13/148) of subjects receiving DAKLINZA and sofosbuvir and was associated with reduced SVR12 rates (Table below). Polymorphisms detected at other NS5A resistance-associated positions were not associated with reduced SVR12 rates; these polymorphisms included M28V (n=1), A30K/S/T/V (n=14), P58R/S (n=3), and S62-any (n=66). Polymorphisms at positions associated with sofosbuvir resistance or exposure (defined as any change from reference at NS5B positions L159, S282, C316, L320, or V321) were not detected in the baseline NS5B sequence of any subject (n=150) in ALLY-3 by population-based sequencing. Phylogenetic analysis of NS5A sequences indicated that all subjects with available data (n=148) were infected with HCV subtype 3a.
Based on resistance patterns observed in cell culture replicon studies and HCV genotype 3-infected subjects, cross-resistance between daclatasvir and other NS5A inhibitors is expected. Cross-resistance between daclatasvir and other classes of direct-acting antivirals is not expected. The impact of prior daclatasvir treatment experience on the efficacy of other NS5A inhibitors has not been studied. Conversely, the efficacy of DAKLINZA in combination with sofosbuvir has not been studied in subjects who have previously failed treatment with regimens that include an NS5A inhibitor.
Safety information was available for approximately 1,900 patients with HCV treated with the recommended dose of DAKLINZA in combination with other anti-HCV drugs in clinical trials. The most common side effects of DAKLINZA with sofosbuvir were fatigue (14%), headache (14%), nausea (8%) and diarrhea (5%). All adverse reactions were mild to moderate in severity. One subject experienced a serious adverse event that was considered unrelated to DAKLINZA, and no subjects discontinued therapy for adverse events. Transient, asymptomatic lipase elevations of greater than 3 times the upper limit of normal (ULN) were observed in 2% of subjects in ALLY-3.
DAKLINZA labeling includes a WARNINGS and PRECAUTIONS statement that serious slowing of the heart rate (symptomatic bradycardia) and cases requiring pacemaker intervention have been reported when amiodarone is co-administered with sofosbuvir in combination with another HCV direct-acting antiviral, including DAKLINZA. Co-administration of amiodarone with DAKLINZA in combination with sofosbuvir is not recommended.
The approve product label will be available soon at http://dailymed.nlm.nih.gov/dailymed/, or at drugs@fda
Richard Klein
Office of Health and Constituent Affairs
Food and Drug Administration
Kimberly Struble
Division of Antiviral Products
Food and Drug Administration
Steve Morin
Office of Health and Constituent Affairs
Food and Drug Administration
|
|
| |
| |
|
|
|