| |
New "Recommendations for the management of hepatitis C virus infection among people who inject drugs" but not new......"HCV Test & Treat" / Routine HCV Screening
|
| |
| |
Download the PDF here
HCV Test & Treat......."10 mill IDUs worldwide; 6.6 ever in USA, 770,000 last year"....we need routine HCV screening & "Test & Treat" .......new publication below in Intl Jnl of Drug Policy lays out recommendations for IDUs & HCV treatment.....these were all part of NYC screening program I designed & raised funding for 4 yrs ago.......in NYC we full well understand what is needed, and in many cities around the US we do as well, the problem is restrictions prevent "HCV Test & Treat", the restrictions are at the core of any headway in HCV & deprioritizes treating IDUs. And as I have been writing about recently the federal govt supports the restrictions tacitly because they have done nothing to discuss or address it.....HCV DRUG Wars - The Federal & State Medicaid Deceit, Lies & Scandal.....
Compare Prices - HCV Drug Pricing vs New Cholesterol Drugs - (08/03/15) .......if it sounds like I am frustrated I AM, I have been working on these issues for 20 years & then the restrictions prevent progress!
from Jules: This subject is not new to me or others, certainly not in the USA & my guess not in England either. I have been writing about this for many years, "HCV Test & Treat", this and routine HCV screening should be part of the landscape in the USA, every should be screened but certainly in inner cities and clearly now with the emerging heroin & associated HCV problem among young whites these populations should be routinely screened. However, restrictions to access to treatment prevent a "Test & Treat " Program. It is tiresome because I have been writing about this for years. The NEW PUBLICATION below discusses recommendations for IDUs, these are great but not new- these were all part of the "Check Hep C" Project in NYC which I designed & raised the $2 million in funding for about 4 years ago now, this was the first inner city large scale HCV screening project, I started the discussion of Patient Navigators for this project now of course this term is ubiquitous.
------------------------------
Global HCV Report HCV/IDUs/PWID - HCV in the USA [New NHANES HCV report] (27% African-Americans, that is double the 13% in the US population), PWIDs in the USA (6.6 million ever - 770,00 in last year injected drugs). Latest Report on Latinos in USA with HCV: men and women of Puerto Rican background in the Bronx had the highest HCV prevalence (14.2% and 4.1%, respectively)..... http://www.natap.org/2014/HCV/071614_04.htm
-----------------
Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews - 10 Million IDUs HCV+ Globally - 6.4 HBV+......http://www.natap.org/2011/newsUpdates/072811_01.htm........Our global systematic review suggested that around 10.0 million IDUs are HCV positive and around 1.2 million are HBsAg positive.....About 10.0 million (range 6.0-15.2) IDUs worldwide might be anti-HCV positive. China (1.6 million), USA (1.5 million), and Russia (1.3 million) had the largest such populations......inadequate capacity to deliver effective interventions compromises outcomes everywhere
IDUs / Barriers to Care.....http://natap.org/2014/HCV/080814_03.htm........HCV positive injection drug users and linkage to care: a missed opportunity........Of the 188 past or current PWID who completed our questionnaire, 95% reported having received at least 1 HCV test in the past......66% reported their HCV status as positive. Of the HCV positive participants, 36% were uninsured, 62% had never seen an HCV specialist, and only 15% had ever received treatment. Multiple subjective access to care barriers were reported by participants: no insurance 30%; can't afford copay 30%; can't afford transportation 40%; treatment will make me sick 90%; fear of liver biopsy 40%; feel fine without treatment 30%; fear of judgement by dr 40%.
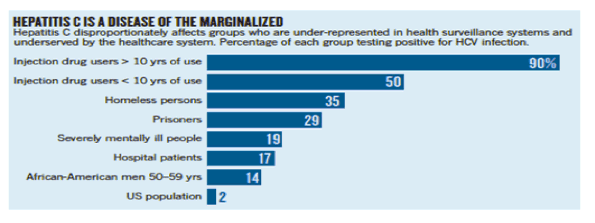
Eliminating HCV " Test & Treat" - HCV is a Disease of the Marginalized: homeless, IDUs, immigrants, African-Americans, Latinos ........Barriers to HCV Screening/Care among IDUs: "Perceptions of drug users regarding Hepatitis C screening and care: a qualitative study"......http://www.natap.org/2013/HCV/122713_04.htm
.......http://www.natap.org/2014/HCV/052814_02.htm
EASL: IMPACT OF NEW DAA-CONTAINING REGIMENS ON HCV TRANSMISSION AMONG INJECTING DRUG USERS (IDUS): A MODEL-BASED ANALYSIS (ANRS 12376)...."Test & Treat", Eliminating HCV.......http://www.natap.org/2014/EASL/EASL_87.htm
........Treatment as Prevention" for IDUs could significantly reduce, perhaps eliminate HCV, end HCV, as a contagious disease among the IDU community, but also contribute to eliminating HCV all together, if all the stakeholders can come together to collaborate.......combine an improvement of testing, link to care, adherence to treatment and change in recommendations to treat as soon as possible was the only strategy that led to a high decrease of prevalence and of the number of complications
Estimating the Number of Persons Who Inject Drugs in the United States by Meta-Analysis to Calculate National Rates of HIV and Hepatitis C Virus Infections ......http://www.natap.org/2014/HCV/053014_01.htm......Using data from national population-based U.S. surveys, we estimated that persons who ever injected drugs comprised 2.6% (CI: 1.8%-3.3%) of the U.S. population. This represents approximately 6,612,488 million PWID (range: 4,583,188-8,641,788) aged 13 years or older in 2011. Although PWID comprise 3% or less of the U.S. population, they account for 22% of all persons living with HIV infection [2]. Our estimates also quantified the recognized disparity of HIV disease rates among black and Hispanic/Latino male and female PWID when compared with white male and female PWID.
HCV in India at EASL: IDUs, Burden, Limited Access......
http://www.natap.org/2014/EASL/EASL_117.htm
---------------
Recommendations for the management of hepatitis C virus infection among people who inject drugs
International Journal of Drug Policy July 2015
Jason Grebely a,*, Geert Robaeys b,c,d, Philip Bruggmann e, Alessio Aghemo f, Markus Backmund g,h, Julie Bruneau i, Jude Byrne j, Olav Dalgard k, Jordan J. Feld l, Margaret Hellardm,n, Matthew Hickman o, Achim Kautz p, Alain Litwin q, Andrew R. Lloyd r, Stefan Mauss s, Maria Prins t,u, Tracy Swan v, Martin Schaefer w,x, Lynn E. Taylor y, Gregory J. Dore a on behalf of the International Network for Hepatitis in Substance Users
Given the burden of HCV-related disease among PWID, strategies to enhance HCV testing, linkage to care, assessment, treatment and prevention of HCV reinfection in this group are urgently needed.
Treatment management
Recommendations
· (1) HCV treatment for PWID should be considered on an individualized basis and delivered within a multidisciplinary team setting (Class I, Level A).
· (2) Access to harm reduction programs, social work and social support services should be a component of HCV clinical management (Class I, Level A).
· (3) Peer-based support should be evaluated as a means to improve HCV clinical management (Class I, Level B).
examples of strategies that have been successful for enhancing assessment, adherence or SVR include hospital-based and primary care-based integrated care, community-based tele-health, nurse-led education, psychoeducation, directly observed therapy, peer support groups and peer support workers. The key basis for effective HCV clinical management within all these settings is access to a multidisciplinary team, generally including clinician and nursing clinical assessment and monitoring, drug and alcohol services, psychiatric services, and social work and other social support services (including peer support, if available). Combined social interventions should target on housing, stigma reduction, criminalisation and health care delivery
-------------------------
Reinfection following successful HCV treatment
Recommendations
· (1) PWID should not be excluded from HCV treatment on the basis of perceived risk of reinfection (Class I, Level B).
· (2) Harm reduction education and counselling should be provided for PWID in the context of HCV treatment to prevent HCV reinfection following successful treatment (Class I, Level B).
· (3) Following SVR, monitoring for HCV reinfection through annual HCV RNA assessment should be undertaken on PWID with ongoing risk behaviour (Class I, Level B).
---------------------
HIV/HCV co-infection
Recommendations
· (1) HCV-infected PWID should be screened for HIV (Class I, Level C).
· (2) The accelerated HCV disease progression in HIV/HCV should be considered in treatment decision-making; HCV treatment should be prioritized in HIV/HCV patients regardless of fibrosis stage (Class I, Level B).
· (3) HIV/HCV-coinfected PWID should be treated and retreated with the same DAA regimens as HCV-monoinfected persons, after recognizing and managing interactions with antiretroviral medications (Class I, Level B).
· (4) Early introduction of cART should be offered to all people with HIV infection (Class I, Level A).
· (5) Potential drug-drug interactions between HIV, HCV and OST need to be considered. Consultation with a frequently updated database/prescribing information is indicated (Class I, Level A).
----------------
Highlights
· HCV testing, linkage to care and treatment is low among PWID.
· New interferon-free HCV therapies have the potential to enhance HCV care.
· HCV treatment is safe and effective among PWID.
· HCV testing, linkage to care and treatment should be offered to all PWID.
· These recommendations provide a framework to enhance HCV care among PWID.
HCV prevalence among PWID populations ranges from <20% to >80% (mid-point HCV estimate: 67% antibody positive; 50% RNA positive), with a global estimate of 10 million HCV antibody positive PWID (7.5 million with chronic HCV infection) (Hagan et al., 2008, Nelson et al., 2011). HCV genotypes 1a, 1b, and 3a are common among PWID (Pybus, Cochrane, Holmes, & Simmonds, 2005), 4d is common among PWID in Europe (van Asten et al., 2004), and 6 in Southeast Asia (Sievert et al., 2011). HCV incidence among PWID also varies considerably from 2% to 66% per annum (Hagan et al., 2008, Page et al., 2013, Wiessing et al., 2014). Studies on time to HCV infection have demonstrated highest incidence in the initial years of injecting (Hagan et al., 2008, Roy et al., 2009).
----------------------------
Pre-therapeutic assessment
Recommendations
· (1) Pre-therapeutic education should include discussions of HCV transmission, risk factors for fibrosis progression, treatment, reinfection risk and harm reduction strategies (Class I, Level B).
· (2) Pre-therapeutic assessment should include an evaluation of housing, education, cultural issues, social functioning and support, finances, nutrition and drug and alcohol use. PWID should be linked into social support services, and peer support if available (Class I, Level B).
· (3) Models of HCV care integrated within addiction treatment and primary care health centers, as well as prisons, allow successful pre-therapeutic assessment (Class I, Level B).
· (4) Peer-driven interventions delivered within OST settings may lead to higher rates of treatment initiation and should be offered, if available (Class IIa, Level C).
· (5) Care coordination in conjunction with behavioural interventions can increase likelihood of PWIDs being evaluated and initiating treatment and should be offered, if available (Class I, Level B).
---------------------------
Indications for treatment
Recommendations
· (1) PWID should receive HCV assessment, with treatment decisions based on an individualised evaluation of social, lifestyle, and clinical factors (Class I, Level B).
· (2) Treatment is recommended for PWID with chronic HCV infection (Class I, Level A).
----------------------
PEG-IFN and DAA-based treatment: treatment recommendations
Recommendations
· (1) Evaluation of safety and efficacy of interferon-free DAA regimens is required in PWID (Class I, Level C).
· (2) Sofosbuvir, sofosbuvir/ledipasvir, paritaprevir/ritonavir/ombitasvir/dasabuvir, daclatasvir, and simeprevir can be used in PWID on OST (Class I, Level B).
· (3) The decision to institute therapy in PWID should be based on the availability of agents locally and individual disease characteristics of infected persons. For regions without access to interferon-free DAA therapy, PWID with early liver disease should generally be advised to await access to interferon-free DAA regimens. For those with access to highly effective interferon-free DAA therapy, anyone with chronic HCV infection should be considered for therapy, taking into account social circumstances, adherence and medical and social co-morbidities (Class I, Level B).
· (4) DAA therapy does not require specific methadone and buprenorphine dose adjustment, but monitoring for signs of opioid toxicity or withdrawal should be undertaken (Class I, Level B).
-----------------------
Impact of drug use on adherence and SVR
Recommendations
· (1) Adherence assessments should consider missed doses and treatment discontinuation (Class I, Level B).
· (2) PWID should be counselled on the importance of adherence in attaining an SVR (Class I, Level A).
· (3) A history of IDU and recent drug use at treatment initiation are not associated with reduced SVR and decisions to treat should be made on a case-by-case basis (Class I, Level B).
· (4) PWID with ongoing social issues, history of psychiatric disease and those with more frequent drug use during therapy are at risk of lower adherence and SVR and need to be monitored closely during therapy (Class I, Level B).
----------------------------
Impact of mental health on adherence and SVR
Recommendations
· (1) Pre-treatment assessment should include an evaluation of previous or current psychiatric illness, engagement with a drug and alcohol counselor or psychiatrist and discussions around potential treatment options (Class I, Level A).
· (2) In cases of acute major and uncontrolled psychiatric disorders, a pre-treatment psychiatric assessment is recommended (Class IIa, Level C).
· (3) In case of relevant psychiatric co-morbidities with an increased risk for interferon-associated psychiatric side effects interferon-free DAA therapy should be considered (Class IIb, Level C).
----------------------------
Epidemiology and prevention of HCV
Recommendations
· (1) PWID should be provided with appropriate access to OST and sterile drug injecting equipment as part of widespread comprehensive harm reduction programs (Class I, Level B).
· (2) PWID should be offered HCV treatment, given they are at elevated risk of HCV transmission and successful treatment may yield transmission reduction benefits (Class IIa, Level C).
Ageing cohorts of PWID with chronic HCV and low treatment uptake are currently leading to an increasing burden of HCV related morbidity and mortality (Grebely and Dore, 2011, Hajarizadeh et al., 2013). In several countries where PWID are the major population affected by HCV, 20-25% of deaths among HCV-infected individuals are from liver disease and 15-30% are from drug-related causes (Grebely & Dore, 2011).
Recommendations
· (1) PWID should be counselled to moderate alcohol intake, or abstain if evidence of advanced liver disease (Class I, Level A).
· (2) Cessation of injecting is not required to limit HCV disease progression (Class IIa, Level C).
----------------------
Recommendations
· (1) An anti-HCV test is recommended for HCV testing among PWID, and if the result is positive, current infection should be confirmed by a sensitive RNA test (Class I, Level B).
· (2) PWID who are anti-HCV negative should be routinely and voluntarily tested for HCV antibodies/RNA and if negative, every 12 months. Testing should also be offered following a high risk injecting episode (Class IIa, Level B).
· (3) PWID who are anti-HCV antibody positive and HCV RNA negative (through spontaneous or treatment-induced clearance) should receive regular HCV RNA testing, every 12 months or following a high risk injecting episode (Class IIa, Level B).
-----------------------
Recommendations
· (1) Non-invasive assessments have a reduced risk and greater acceptance than liver biopsy, may enhance HCV screening and disease assessment among PWID, and should be offered, if available (Class I, Level B).
· (2) Combining multiple non-invasive assessments is recommended, when possible (Class I, Level B).
-------------------
HCV treatment in prisons
Recommendations
· (1) Screening and assessment for HCV should be offered to PWID in custody (Class IIa, Level C).
· (2) Antiviral treatment for PWID in custody is feasible and clinically effective and should be offered to PWID in custody (Class IIa, Level B).
|
|
| |
| |
|
|
|