| |
Optimism for patients with genotype 4 HCV infection: Clinical trials with direct-acting antivirals finally available - Editorial
|
| |
| |
Download the PDF here
Journal of Hepatology May 2015
Tarik Asselah
Service d'Hepatologie, PMAD Hopital Beaujon, UNITY, INSERM, UMR1149, Team Viral hepatitis, Centre de Recherche sur
l'inflammation, Labex INFLAMEX, Universite Denis Diderot Paris 7, Clichy Cedex, France
This Editorial discusses three recent original papers related to direct-acting antivirals (DAAs) for the treatment of chronic genotype (GT) 4 HCV infection, published in this issue of the Journal of Hepatology [[1], [2], [3]].
Why is HCV genotype 4 (G4) a major unmet medical need?
Among the 170 million HCV-infected subjects worldwide (around 34 million) HCV-G4 accounts for approximately 20% of those infected. This population accounts for most new infections, with limited access to therapy. While in the US HCV-G4 is responsible for 1-2% of HCV infection, in the Middle East and Sub-Saharan African regions it is the main cause of HCV infection, with Southern Europe also detecting an increase in cases [4]. However, only limited country-specific estimates of HCV prevalence are available to guide decision making treatments. In Egypt, where the prevalence of HCV is the highest in the world, the reuse of glass syringes during the parenteral therapy campaigns to control endemic schistosomiasis is widely held to be responsible for a large number of iatrogenic transmissions [5].
HCV-G4 has been considered "difficult to treat" with pegylated interferon (PegIFN) and ribavirin (RBV) treatment, with sustained virological response (SVR) rates around 50%. A major predictor of response to PegIFN-RBV therapy in patients with HCV-G4 was the IL28B genotype [6]. SVR rates for IL28B rs12979860 CC patients ranged from more than 80% to around 30% for TT patients. Egyptian patients infected with HCV-G4 treated with PegIFN-RBV in Europe responded better than French/European or African patients infected with the same genotype [6]. An overall better response was observed in patients infected with the HCV-G4 subtype 4a, which was the predominant subtype among patients infected in Egypt compared to patients from Sub-Saharan Africa. The distribution of IL28B polymorphism in different ethnicities may be the explanation for this difference in terms of SVR: rs12979860 CC is more frequent in Egyptians than Caucasians, and even more than Black-Africans [[6], [7]].
Available data gives new hope with DAAs for HCV-G4
The introduction of all-oral, IFN-free regimens that combine DAA agents has significantly advanced the treatment of HCV, especially for patients with HCV GT1 infection [8]. High efficacy rates (greater than 95%), low rates of treatment discontinuation, and favorable adverse event (AE) profiles have been demonstrated with multiple regimens, both with and without RBV. However, data on efficacy and safety of DAA in patients with HCV-G4 infection are limited.
Three recent original papers reporting the efficacy of DAAs for the treatment of chronic HCV-G4 infection, have been published in the Journal of Hepatology [[1], [2], [3]].
In a first article, the authors conducted an open-label phase 2 study to assess the efficacy and safety of sofosbuvir (SOF) in combination with RBV in patients of Egyptian ancestry chronically infected with HCV-G4 (ClinicalTrials.gov Identifier NCT01713283) [1]. Treatment-naïve (n = 30) and previously treated patients (n = 30) with HCV-G4 were randomly allocated in a 1:1 ratio to receive SOF 400 mg and weight-based RBV for 12 or 24 weeks. SVR12 was achieved by 68% of patients (95% CI, 49-83%) in the 12 week group and 93% of patients (95% CI, 77-99%) in the 24 week group. This study suggested that treatment with 24 weeks of SOF plus RBV is effective and well-tolerated in patients with HCV-G4 infection. No viable resistance-associated variants were detected in any of the patients who did not achieve SVR. Overall and in nearly every patient subgroup, patients receiving 24 weeks of treatment had substantially higher SVR rates than those receiving 12 weeks of treatment.
In this study, the overall number of patients is small. Especially for difficult to treat patients with cirrhosis, efficacy of this regimen will only be defined by future trials. There is a very large ongoing national Egyptian program with the use of this regimen and we hope data will soon be available.
In a second manuscript, the authors evaluated the efficacy and safety of simeprevir (SMV) with PegIFN-α-2a/RBV in patients with chronic HCV-G4 infection in an open-label, single-arm study (RESTORE; NCT01567735) [2]. Among 107 patients included; treatment-naïve (n = 35) and prior relapse patients (n = 22) received SMV 150 mg once daily (QD) + PegIFN/RBV (12 weeks), followed by PegIFN/RBV alone (12 or 36 weeks, response-guided [HCV RNA <25 IU/ml detectable/undetectable at week 4 and <25 IU/ml undetectable at week 12]). Prior non-responders (partial, n = 10; null, n = 40) received SMV/PegIFN/RBV (12 weeks), followed by PegIFN/RBV for 36 weeks. Overall, 65.4% (70/107) of patients achieved SVR12 (82.9% [29/35] treatment-naïve; 86.4% [19/22] prior relapsers; 60.0% [6/10] prior partial responders; 40.0% [16/40] prior null responders). In treatment-naïve and prior relapser patients fulfilled response-guided criteria for 24 weeks of treatment (88.6% [31/35] and 90.9% [20/22]), SVR12 rates were high: 93.5% [29/31] and 95.0% [19/20], respectively. Overall on-treatment failure and relapse rates were 23.4% (25/107) and 14.6% (12/82), respectively.
AEs were mainly grade 1/2; serious AEs were infrequent (4.7%) and considered unrelated to SMV. The SVR12 rate in patients with METAVIR score F4 was 46.7% (14/30). It should be noted that most of these patients [63%; 19/30] were prior partial and null responders. The SVR12 rate in patients with METAVIR score F0-F2 was 76.3% (45/59) compared with 66.7% (10/15) in patients with F3; 37% (22/59) of patients with METAVIR F0-F2 and 13% (2/15) of those with F3 were prior partial or null responders. The effectiveness of protease inhibitor-based regimens in combination with PegIFN/RBV may be limited in patients who are non-responders to previous PegIFN/RBV therapy. Higher SVR rates are observed in these patients with IFN-free combinations. The authors concluded that the efficacy and safety of SMV 150 mg QD for 12 weeks with PegIFN/RBV in treatment-naïve or experienced patients with chronic HCV-G4 infection were in line with previous reports for HCV-G1 infection. These results support the use of an RGT-based approach to individualise the duration of treatment in HCV-G4-infected patients. Shortening treatment may be beneficial in these patients, as it would reduce overall drug exposure and minimise therapy costs. A 12 week trial with SMV and PegIFN/RBV is ongoing (NCT01846832).
In the third study, the authors explored the oral combination of daclatasvir (NS5A inhibitor), asunaprevir (NS3 protease inhibitor), and beclabuvir (non-nucleoside NS5B polymerase inhibitor) in a randomized, open-label, phase 2a study, including 21 HCV-G4 naïve patients (NCT01455090) [3]. The patients (N = 21) were enrolled at nine sites in the USA as an expansion of a larger study, and were randomized 1:1 to receive a twice-daily oral regimen comprising 75 mg or 150 mg of beclabuvir, each with daclatasvir (30 mg) and asunaprevir (200 mg), for 12 weeks with 48 weeks of post-treatment follow-up. HCV RNA decline was rapid: median (range) log10 change from baseline at day 7 was -4.39 (-4.91, -2.95) IU/ml in the 75 mg beclabuvir group and -4.01 (-5.03, -3.47) IU/ml in the 150 mg group. All patients had <25 IU/ml by week 2 on-treatment. Two patients, one in each treatment arm, were missing data at post-treatment week 12 but were confirmed to have <25 IU/ml of HCV RNA at their analysis, and by 100% of patients in both arms by the imputed analysis. Concordance between SVR12 and SVR24 was 100% in the 9 patients in each arm with available HCV RNA data at both these post-treatment time points. Although baseline RAVs in NS5A were observed in just under half the patients assessed, there were no on-treatment virologic failures, and no post-treatment relapses through the primary end point or in any patient for whom post-SVR12 data were available at time of analysis. There were no notable safety or tolerability issues, consistent with previous data for this regimen with genotype 1. The use of a fixed-dose combination tablet is currently under phase 3 evaluation.
What are the other published data (SVR) with DAAs for HCV-G4?
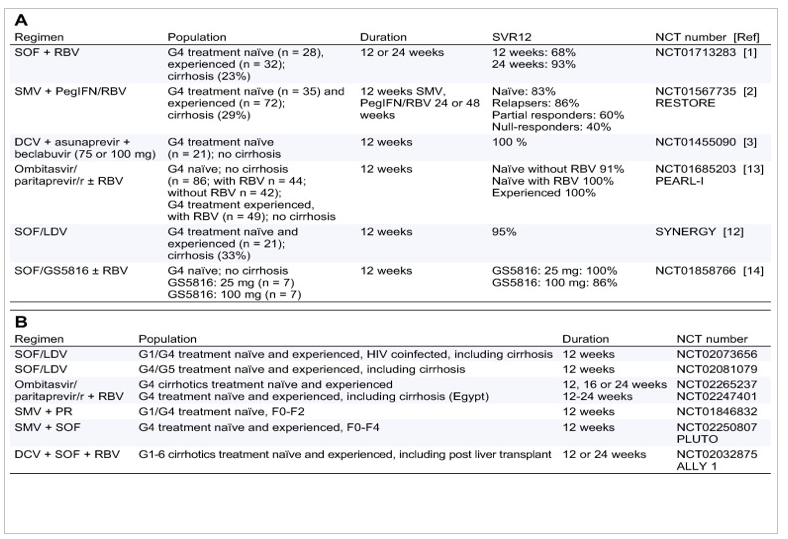
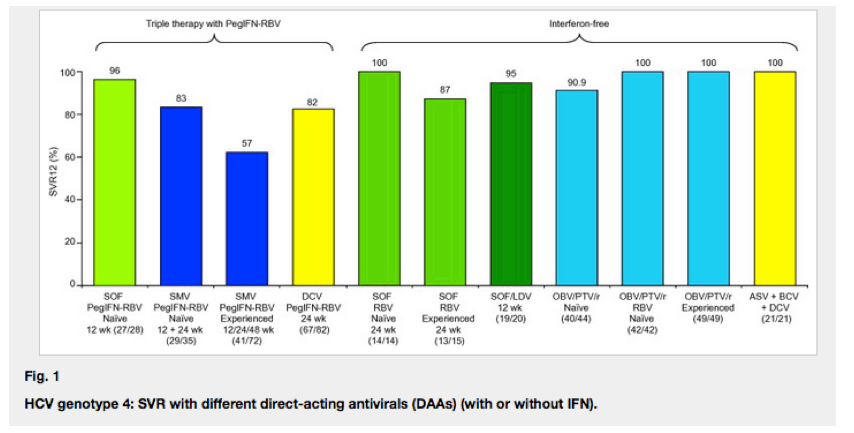
Recent data from several studies with or without PegIFN have become recently available [[9], [10], [11], [12], [13], [14]], and these data are summarized in Table 1A. SVR with different DAAs (with or without IFN) are illustrated in Fig. 1. Finally, the main limitations of these clinical trials on HCV-G4 are their small sample size and the relatively mild stage of liver diseases included in patients. In real-life, we treat first the patients with advanced fibrosis (F3/F4) and we need particularly data for this population. Moreover, the huge number of HCV genotype 4 subtypes may be of importance in terms of antiviral activities of DAAs (NS5A inhibitors and especially non-NUC NS5B inhibitors).
Table 1(A) Available studies on direct-acting antivirals (DAAs) with or without PegIFN for HCV-G4. (B) Ongoing clinical trials including HCV-G.
Because of the limited available data, it is difficult to provide guidelines or guidance to help physicians with their patients. The European Association for the Study of the Liver (EASL) guidelines include 6 treatment options for treatment-naïve and PegIFN/RBV experienced patients with HCV-G4 infection [15]:
· 1.SOF + PegIFN-RBV for 12 weeks.
· 2.SIM + PegIFN-RBV (SIM: for 12 weeks, PegIFN-RBV: 24 weeks in naïve and relapsers, 48 weeks in prior partial and null responders. Stop treatment if HCV RNA ≥25 IU/ml at treatment week 4, 12 or 24).
· 3.DCV + PegIFN-RBV for 12 weeks. Extended for additional 12 weeks in patients whose HCV RNA not <25 IU/ml at week 4 and undetectable at week 10. PegIFN-RBV continued alone in patients whose RNA <25 IU/ml at week 4 and undetectable at week 10.
· 4.SOF + RBV for 24 weeks in IFN intolerant or ineligible patients.
· 5.SOF + SIM (±RBV) for 12 weeks.
· 6.SOF + DCV (±RBV) for 12 weeks in treatment-naïve, and 24 weeks in treatment experienced patients.
These guidelines were released before the approval of two additional regimens, SOF plus ledipasvir and the combination of paritaprevir/r plus ombitasvir plus RBV which have shown high SVR rates.
Finally, there is optimism for patients with genotype 4 HCV infection, with several promising ongoing trials that are summarized in Table 1B [[16], [17], [18]]. With these excellent data, the next steps will be to improve screening and access to therapy.
|
|
| |
| |
|
|
|