| |
Incidence of sexually transmitted hepatitis C virus infection in HIV-positive MSM: a systematic review and meta-analysis - HCV in MSM High-Risk Groups - need for targeted hepatitis C prevention and testing campaigns for this group
|
| |
| |
Download the PDF here
"These data indicate that there exists a subgroup of HIV-positive MSM with recurring sexual exposure to HCV in whom the rates may begin to approach the risk of HCV infection among PWID......HCV seroconversion reported on associations with potentially causal exposures (sexual risk behavior...in the New York City case-control study [48], the adjusted odds ratio (AOR) for receptive anal intercourse without a condom, with ejaculation was 23.0 (95% CI 2.2-243.8) and the proportion of HCV infections in the cases that were attributable to this behaviour was 95.7%.......Reinfection postsuccessful HCV treatment (n = 2 studies) was 20 times higher than initial seroconversion rates.......Results from phylogenetic analyses suggest networks of HCV transmission among these men.....in one study, five HIV positive MSM were reinfected more than once following SVR, and multiple reinfections were also observed in men who experienced spontaneous clearance [49]. Another study reported on two cases of HCV super-infection among HIV/HCV-coinfected MSM engaging in high risk sexual behavior"....[although the rates of new HCV in MSM & reinfection are lower than that seen in PWIDs it is a growing trend & there exists a subgroup of HIV+ MSM & MSN not HIV-infected with recurring sexual exposure through high-risk behavior which includes unprotected anal intercourse & substance abuse which increases risky sex behavior, at parties in NYC, Miami and other major cities]
from Jules: this is the latest report from NATAP on HCV in MSM. The NY authors report in Table 2 below on data from NY from Daniel Fierer, here is a link to the full report that was published in CDC MMWR: http://www.natap.org/2011/HCV/072211_01.htm ....."This report suggests high-risk sexual behavior as a cause of HCV transmission among HIV-infected MSM in New York City. Unprotected receptive anal intercourse with ejaculation and sex while high on methamphetamine were the most important predictors of HCV infection. Results from phylogenetic analyses suggest networks of HCV transmission among these men. The findings of high-risk sex, concurrent noninjection-drug use, and phylogenetic clustering are similar to those observed among cohorts of HIV-infected MSM with HCV infection in Northern Europe and Australia (4). A notable finding from this study and those in other countries is the association of noninjection, recreational drug use (e.g., methamphetamine use) with the acquisition of HCV infection"
HCV Tripled Among HIV+ MSM in NYC, Earlier Death Reported by NYC......http://www.natap.org/2015/HCV/071015_02.htm....."There was a significant upward trend in the proportion of HCV infections reported among people with HIV who are MSM. In 2000, 7% of HCV reports among people with HIV were MSM; in 2010, 24% were MSM....The increase in the percentage of HCV reports among persons with HIV that have MSM as a risk factor indicate a need for targeted hepatitis C prevention and testing campaigns for this group"
-------------
Incidence of sexually transmitted hepatitis C virus infection in HIV-positive MSM: a systematic review and meta-analysis
"......pooled rate of HCV seroconversion was 0.5/100 person-years.......These HCV incidence rates are still relatively low compared with people who inject drugs (PWID). Indeed, the highest rates of HCV infection in HIV-positive MSM do not reach the lower bound of the range in PWID (5-60/100 person-years)......data show an upward trend beginning in about 1995. If the trend identified in the meta-regression has continued to the present, current incidence may be as high as 1.92/100 person-years, but the predictions also show increasing uncertainty over the past several years......HCV seroconversion reported on associations with potentially causal exposures (sexual risk behavior...in the New York City case-control study [48], the adjusted odds ratio (AOR) for receptive anal intercourse without a condom, with ejaculation was 23.0 (95% CI 2.2-243.8) and the proportion of HCV infections in the cases that were attributable to this behaviour was 95.7%......These data indicate that there exists a subgroup of HIV-positive MSM with recurring sexual exposure to HCV in whom the rates may begin to approach the risk of HCV infection among PWID......These HCV incidence rates are still relatively low compared with people who inject drugs (PWID). Indeed, the highest rates of HCV infection in HIV-positive MSM do not reach the lower bound of the range in PWID (5-60/100 person-years) [54]. However, our analysis also showed that the pooled rate of reinfection following successful HCV treatment was 20 times higher than the rate of initial infection in HIV-positive MSM - 11/100 person years - and 2-year cumulative incidence post-SVR in two studies was 25-33%. Indeed, in one study, five HIV positive MSM were reinfected more than once following SVR, and multiple reinfections were also observed in men who experienced spontaneous clearance [49]. Another study reported on two cases of HCV super-infection among HIV/HCV-coinfected MSM engaging in high risk sexual behaviour [55]."
"Reinfection postsuccessful HCV treatment (n = 2 studies) was 20 times higher than initial seroconversion rates. Among the seroconverters, a large proportion of infections were attributable to high-risk behaviours including mucosally traumatic sex and sex while high on methamphetamine.
Conclusion: The high reinfection rates and the attributable risk analysis suggest the existence of a subset of HIV-positive MSM with recurring sexual exposure to HCV. Approaches to HCV control in this population will need to consider the changing epidemiology of HCV infection in MSM.
In this systematic review and meta-analysis that included 13 000 HIV-positive MSM enrolled in 15 studies in urban areas of Europe, the USA, Australia and Asia, the pooled rate of HCV seroconversion was 0.5/100 person-years. The data show an upward trend beginning in about 1995. If the trend identified in the meta-regression has continued to the present, current incidence may be as high as 1.92/100 person-years, but the predictions also show increasing uncertainty over the past several years (95% CI 0.77-4.80). This suggests that additional primary data collection and updated meta-analyses should be a high priority to estimate the current burden of HCV infection.
These HCV incidence rates are still relatively low compared with people who inject drugs (PWID). Indeed, the highest rates of HCV infection in HIV-positive MSM do not reach the lower bound of the range in PWID (5-60/100 person-years) [54]. However, our analysis also showed that the pooled rate of reinfection following successful HCV treatment was 20 times higher than the rate of initial infection in HIV-positive MSM - 11/100 person years - and 2-year cumulative incidence post-SVR in two studies was 25-33%. Indeed, in one study, five HIV positive MSM were reinfected more than once following SVR, and multiple reinfections were also observed in men who experienced spontaneous clearance [49]. Another study reported on two cases of HCV super-infection among HIV/HCV-coinfected MSM engaging in high risk sexual behaviour [55]. These data indicate that there exists a subgroup of HIV-positive MSM with recurring sexual exposure to HCV in whom the rates may begin to approach the risk of HCV infection among PWID.
REINFECTION: Four studies reported on HCV reinfection in HIV positive MSM following HCV treatment and SVR. Two studies provided incidence density estimates of reinfection, 15.2/100 person-years in Amsterdam [50], and 9.6/100 person-years in London [49]. The pooled rate for these two studies was 11.41/100 person-years (95% CI 7.36-17.68). Both of these studies also reported high 2-year cumulative rates of reinfection (25-33%). Two other studies estimated that 16-28% were reinfected post- SVR, but the amount of observation time was not given [51,52].
"......HCV seroconversion reported on associations with potentially causal exposures (sexual risk behavior.......
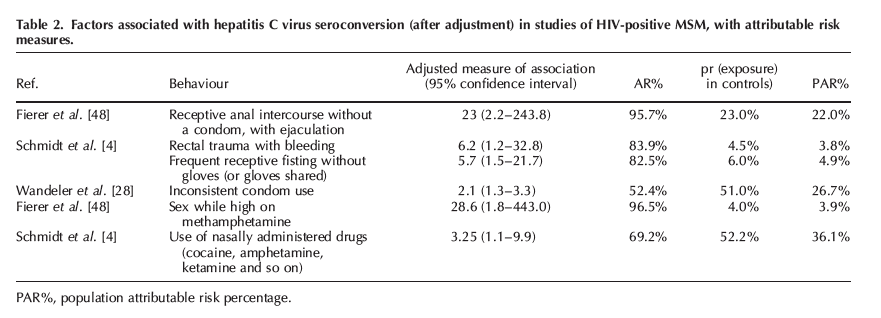
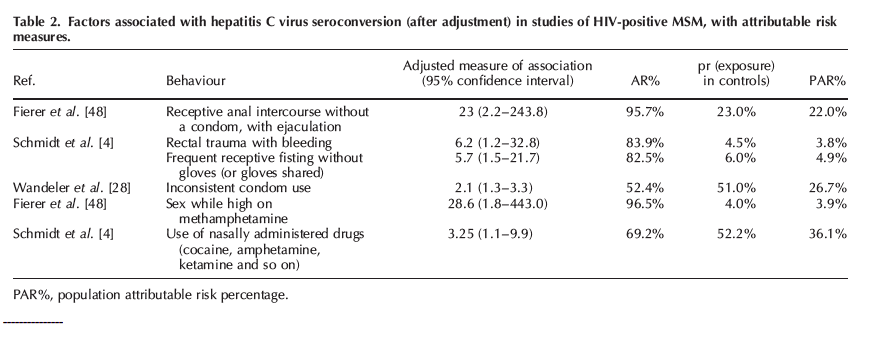
As summarized in Table 2, in the New York City case-control study [48], the adjusted odds ratio (AOR) for receptive anal intercourse without a condom, with ejaculation was 23.0 (95% CI 2.2-243.8) and the proportion of HCV infections in the cases that were attributable to this behaviour was 95.7%.
The proportion of infections in the underlying HIV-positive MSM population attributable to receptive anal intercourse without a condom, with ejaculation (the PAR%) was 22%. In the German study [4], the AOR for rectal trauma during sex with bleeding was 6.2 (95% CI 1.2-32.8), the AR% was 71% and (owing to a low prevalence of this exposure in the underlying population) the PAR% was 3.8%. Frequent receptive fisting without gloves (or gloves shared) was associated with a six-fold excess risk of HCV seroconversion (95% CI 1.5-21.7), the AR% was 83% and the PAR% was also relatively low at 5%. Two studies examined noninjection drug use. These include Fierer et al. [48], wherein sex while high on methamphetamine was associated with a 28.6-fold elevated risk of HCV infection, but the PAR% was only 4% because of low prevalence of this exposure in controls. The use of inhaled drugs was associated with HCV infection in study in Germany, and because half of the controls reported this exposure, the PAR% was 36%. None of the included studies reported statistically significant adjusted estimates of the association between HIV-related factors (CD4?, HIV viral load or antiretroviral therapy) and seroconversion."
---------------
Incidence of sexually transmitted hepatitis C virus infection in HIV-positive MSM: a systematic review and meta-analysis.
AIDS Post Acceptance Aug 7 2015
Hagan, Holly; Jordan, Ashly E.; Neurer, Joshua; Cleland, Charles M.
Center for Drug Use and HIV Research, College of Nursing, New York University, New York, New York, USA.
Correspondence to Holly Hagan, College of Nursing, New York University, 433 1st Avenue, New York, NY 10001, USA
Abstract
Objective: The epidemiology of the incidence of sexually transmitted hepatitis C virus (HCV) infection in HIV-positive MSM is only partially understood. In the presence of HIV, HCV infection is more likely to become chronic and liver fibrosis progression is accelerated.
Design: A systematic review and meta-analysis was used to synthesize data characterizing sexually transmitted HCV in HIV-positive MSM.
Methods: Electronic and other searches of medical literature (including unpublished reports) were conducted. Eligible studies reported on HCV seroconversion or on reinfection postsuccessful HCV treatment in HIV-positive MSM who were not injecting drugs. Pooled incidence rates were calculated using random-effects meta-analysis, and meta-regression was used to assess study-level moderators. Attributable risk measures were calculated from statistically significant associations between exposures and HCV seroconversion.
Results: More than 13 000 HIV-positive MSM in 17 studies were followed for more than 91 000 person-years between 1984 and 2012; the pooled seroconversion rate was 0.53/100 person-years. Calendar time was a significant moderator of HCV seroconversion, increasing from an estimated rate of 0.42/100 person-years in 1991 to 1.09/100 person-years in 2010, and 1.34/100 person-years in 2012. Reinfection postsuccessful HCV treatment (n = 2 studies) was 20 times higher than initial seroconversion rates. Among the seroconverters, a large proportion of infections were attributable to high-risk behaviours including mucosally traumatic sex and sex while high on methamphetamine.
Conclusion: The high reinfection rates and the attributable risk analysis suggest the existence of a subset of HIV-positive MSM with recurring sexual exposure to HCV. Approaches to HCV control in this population will need to consider the changing epidemiology of HCV infection in MSM.
Introduction
Since 2000, there have been multiple reports of outbreaks of sexually transmitted acute hepatitis C virus (HCV) infection in HIV-positive MSM in urban areas of North America, Europe, Australia and Asia [1,2]. Acute HCV infection is more likely to become persistent in the presence of HIV, and liver fibrosis progression in chronic HCV infection is more rapid even in patients with undetectable HIV viral loads [3]. The evidence points to blood as the medium of sexual HCV exposure in these cases [4-9]. Several factors facilitate excess HCV transmission in HIV-positive MSM compared with other populations in which sexual transmission is very rare [10]. HCV viral loads are higher in semen and blood in the presence of HIV, and this may increase the likelihood that infectious carriers will transmit HCV infection [11,12]. Mucosally traumatic sexual practices, erosive genital lesions associated with sexually transmitted infections (STIs) and increased sexual disinhibition and prolonged periods of sexual activity related to the use of stimulant drugs may cause blood exposure during sex [8,10,13].
The purpose of this systematic review and meta-analysis was to synthesize data characterizing the incidence of sexual HCV transmission in HIV-positive MSM and to examine study-level influences on HCV seroconversion rates including calendar time and risk of selection bias. In addition, data on individual risk behaviours were assessed in terms of the strength of the association with HCV seroconversion and the factor's contribution to new HCV infections in the HIV-positive MSM population. Rates of HCV reinfection following successful treatment were also synthesized. Extensive knowledge exists regarding HCV transmission via illicit drug use related exposures, so the examination of drug use in the cause of HCV infection in HIV-positive MSM was limited to its role as a facilitator of transmission via sex-related exposures.
Results
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses [30]) flow diagram describes each phase of this study's systematic review of HCV incidence among HIV-positive MSM (Fig. 1). A total of 1117 potentially eligible reports were identified through the search of electronic databases; another 14 were located through searches of conference abstracts and other sources. After excluding 352 duplicates, the remaining 779 abstracts were screened and a subset (173) was reviewed as full-text articles. After this screening step, 21 reports of HCV seroconversion [4,7,28,31-48] and four reinfection reports [49-52] were eligible. Reasons for ineligibility are also shown in Fig. 1.
Seventeen of the 21 eligible studies of HCV seroconversion (81%) provided incidence density data (n seroconversions, n person-years) that could be used to calculate pooled rates; three out of 17 were conference abstracts. Two were case-control studies that reported on the association between HCV seroconversion and exposures. Two reports [28,41] were from the Swiss HIV Cohort Study (http://www.shcs.ch/) and two were from the Amsterdam Cohort Study (https://www.amsterdamcohortstudies.org/acsc/index.asp) and so only one estimate of incidence from each cohort [28,47] was included in the calculation of the pooled rate, leaving 15 unique estimates of incidence density. As summarized in Table 1, cohort study periods spanned 1984-2012; this included the testing of stored sera after HCV screening tests became available [53]. Half of the studies were from Europe, four from the USA, three from Asia and two from Australia. All took place in high-income countries and in urban settings. In the majority of studies, convenience sampling was used to select study participants; consecutive sampling was used in six cases. In 55% of reports, participants were recruited from HIV clinics. Other recruitment locations included hospitals, STI clinics,'social venues for MSM' and community-based organizations.
Each study's inclusion criteria are described in Table 1.
Pooled hepatitis C virus seroconversion rates and
study-level moderators
More than 13 000 individuals were followed in 15 unique studies to observe 497 cases of HCV seroconversion over 93 100 person-years using incidence density estimation (Fig. 2). HCV incidence rates ranged from 0.00/100 to 1.40/100 person-years. The pooled incidence rate was 0.53/100 person-years (95% CI 0.49-0.58). In a meta-analysis, selection bias was not a significant moderator of seroconversion rates. Reports using convenience sampling had a pooled rate of 0.65/100 vs. 0.47/100 person-years in reports using consecutive sampling [QM(df=1)=0.68, P=0.41]. Significant residual heterogeneity in incidence remained after taking selection bias into account [QE(df=11)=153.5597, P<0.0001; I2=92.60, 95% CI 83.82-97.51].
Calendar time, however, was a significant moderator of HCV seroconversion rates [QM(df=2)1/47.50, P=0.02]. Figure 3 includes predictions of HCV incidence on the basis of the meta-regression with calendar time as a moderator, including up to the present year. Estimated annual incidence rates ranged from a low of 0.42/100 person-years in 1991 (95% CI 0.23-0.77) to 1.34/100 person-years in 2012 (95% CI 0.76-2.36). Incidence in 2010 (centred) was 1.09/100 person-years (95% CI 0.73-1.61), and the instantaneous rate of increase in log incidence in 2010 was 0.10 per year (95% CI 0.003-0.197). The quadratic term showed no significant degree of curvature in the log incidence trend (P=0.27). Significant residual heterogeneity in incidence remained after taking calendar time into account [QE(df=76)1/4257.85, P<0.0001; I2=68.9, 95% CI 50.53-74.16].
Reinfection following SVR
Four studies reported on HCV reinfection in HIV positive MSM following HCV treatment and SVR. Two studies provided incidence density estimates of reinfection, 15.2/100 person-years in Amsterdam [50], and 9.6/100 person-years in London [49]. The pooled rate for these two studies was 11.41/100 person-years (95% CI 7.36-17.68). Both of these studies also reported high 2-year cumulative rates of reinfection (25-33%). Two other studies estimated that 16-28% were reinfected post- SVR, but the amount of observation time was not given [51,52].
Risk factors for hepatitis C virus seroconversion
Only 10 of the 21 studies of HCV seroconversion reported on associations with potentially causal exposures (sexual risk behaviour, sex while using noninjection drugs, recent sexually transmitted infection or an HIV-related factor such as whether on antiretroviral therapy, CD4? cell count or HIV viral load). However, six of the 10 either did not provide adjusted estimates or included IDUs or non-MSM in the risk factor analysis. This left four studies reporting adjusted associations between the potentially causal exposures and HCV seroconversion in HIV-positive MSM [4,28,41,48]. As summarized in Table 2, in the New York City case-control study [48], the adjusted odds ratio (AOR) for receptive anal intercourse without a condom, with ejaculation was 23.0 (95% CI 2.2-243.8) and the proportion of HCV infections in the cases that were attributable to this behaviour was 95.7% (the AR%).
The proportion of infections in the underlying HIV-positive MSM population attributable to receptive anal intercourse without a condom, with ejaculation (the PAR%) was 22%. In the German study [4], the AOR for rectal trauma during sex with bleeding was 6.2 (95% CI 1.2-32.8), the AR% was 71% and (owing to a low prevalence of this exposure in the underlying population) the PAR% was 3.8%. Frequent receptive fisting without gloves (or gloves shared) was associated with a six-fold excess risk of HCV seroconversion (95% CI 1.5-21.7), the AR% was 83% and the PAR% was also relatively low at 5%. Two studies examined noninjection drug use. These include Fierer et al. [48], wherein sex while high on methamphetamine was associated with a 28.6-fold elevated risk of HCV infection, but the PAR% was only 4% because of low prevalence of this exposure in controls. The use of inhaled drugs was associated with HCV infection in study in Germany, and because half of the controls reported this exposure, the PAR% was 36%. None of the included studies reported statistically significant adjusted estimates of the association between HIV-related factors (CD4?, HIV viral load or antiretroviral therapy) and seroconversion.
Discussion/Conclusion
In this systematic review and meta-analysis that included 13 000 HIV-positive MSM enrolled in 15 studies in urban areas of Europe, the USA, Australia and Asia, the pooled rate of HCV seroconversion was 0.5/100 person-years. The data show an upward trend beginning in about 1995. If the trend identified in the meta-regression has continued to the present, current incidence may be as high as 1.92/100 person-years, but the predictions also show increasing uncertainty over the past several years (95% CI 0.77-4.80). This suggests that additional primary data collection and updated meta-analyses should be a high priority to estimate the current burden of HCV infection.
These HCV incidence rates are still relatively low compared with people who inject drugs (PWID). Indeed, the highest rates of HCV infection in HIV-positive MSM do not reach the lower bound of the range in PWID (5-60/100 person-years) [54]. However, our analysis also showed that the pooled rate of reinfection following successful HCV treatment was 20 times higher than the rate of initial infection in HIV-positive MSM - 11/100 person years - and 2-year cumulative incidence post-SVR in two studies was 25-33%. Indeed, in one study, five HIV positive MSM were reinfected more than once following SVR, and multiple reinfections were also observed in men who experienced spontaneous clearance [49]. Another study reported on two cases of HCV super-infection among HIV/HCV-coinfected MSM engaging in high risk sexual behaviour [55]. These data indicate that there exists a subgroup of HIV-positive MSM with recurring sexual exposure to HCV in whom the rates may begin to approach the risk of HCV infection among PWID.
The attributable risk analysis also supports the notion of a high-risk subset of HIV-positive MSM. A large proportion of infections in the HCV seroconverters were attributable to mucosally traumatic sex and sex while high on methamphetamine. These exposures were infrequent in the nonseroconverters. Ideally, an HCV prevention programme for HIV-positive MSM engaging in high-risk sexual practices would integrate successful behavioural and biomedical approaches similar to currently recommended HIV prevention strategies that combine behavioral risk reduction interventions with preexposure prophylaxis (PrEP). Unfortunately, chemoprophylaxis for HCV has not been developed and is unlikely to be available in the near future, and HIV-PrEP does not protect against sexually transmitted HCV. In fact, there have been recent reports of acute HCV in HIV-negative men engaging in high-risk practices with many on daily PrEP [56].
One of the main factors contributing to high HCV incidence rates among PWID is the high prevalence of infectious carriers (50-80%) in the underlying PWID population [57]. Increasing HCV transmission in HIV positive MSM (and HIV-negative MSM) will similarly raise the prevalence of HCV-RNA in MSM communities and facilitate further spread. The observed increase in HCV incidence parallels a number of other trends in MSM communities, including an increased use of social media geosocial sexual networking applications and its association with STIs, the increase in 'seroadaptive' sexual behaviour that includes unprotected sex between men with the same HIV status and rising rates of STIs and use of 'chemsex' drugs, including by injection [13,58-60].
Recommendations for management of acute HCV in HIV-positive MSM centre on detection through screening for elevated liver enzymes every 3-6 months and providing either early or delayed treatment, that is before or after a 12-week period to determine whether the patient has spontaneously cleared infection [62]. Research on the new direct-acting antiviral (DAA) treatments for acute HCV infection suggest that they may be equally effective in curing HCV in mono infected and HIV-coinfected patients [63]. However, the high rates of reinfection and the cost of treatment with the new DAAs may impact on the feasibility of this approach to HCV control among HIV-positive MSM [64]. The changing epidemiology of HCV in MSM communities, towards increasing incidence and prevalence, will only add to the cost and scope of the problem.
There were several limitations to this study that must be acknowledged. As with all systematic reviews and meta analyses, we were limited by the amount and type of data given in the original studies. There was relatively little reporting of exposure risk across the studies, and in many cases, sample sizes were too small to detect significant associations with HCV after adjustment for confounding. Greater reporting of nonsignificant associations would permit the pooling of individuals across studies, increasing power to detect significant covariates. Selection bias was the chief concern in synthesizing the HCV seroconversion rates, and there was limited description of study methods to clearly identify samples that may have over or underestimated HCV risk. Therefore, our meta-regression may have failed to properly adjust for this source of bias. Our analysis of calendar time as a modifier of incidence relied upon interpolation for 12 of 79 incidence estimates, which may have overestimated the precision of observed trends in incidence over time. However, the significant increase in incidence was also observed in the large primary study that required interpolation [28]. In addition, although the seroconversion rates observed in the individual studies were at least an order of magnitude lower than in studies of PWID, it is conceivable that some PWID may have been inadvertently included in the HIV-positive MSM studies. This would have biased the rates upward.
The multifactorial nature of sexually transmitted HCV in HIV-positive MSM will require a combination approach addressing individual sexual and drug use behavior in the context of a changing epidemiology. A fuller understanding of the causal pathways is needed to identify effective strategies, and lessons learned about HIV prevention in MSM engaging in sexual risk behaviour are a useful starting point. The results of this study suggest that cumulative HCV incidence in HIV positive MSM - the result of initial and reinfection - is perhaps a useful way to represent disease burden and to identify subgroups for targeted intervention.
|
|
| |
| |
|
|
|