| |
Genotype 3 - A Special Genotype
|
| |
| |
Reported by Jules Levin, NATAP
On 30 June, a meeting hosted by BMS was held to discuss and evaluate HCV genotype 3 (GT-3) epidemiology, disease pathogenesis, care and treatment, and treatment guidelines.
I participated in the meeting, along with a selected small group of thought leaders from around the globe. The unique aspects of GT-3 infection were discussed, including its accelerated disease pathogenesis and fibrosis progression, higher hepatocellular carcinoma (HCC) rates, higher risk for developing cirrhosis and higher mortality risk compared with other genotypes. GT-3 patients can experience fatty liver, or steatosis, which may play a role in this accelerated disease progression. Compounding these challenges, GT-3 has proven to be less responsive to the interferon (IFN)-free direct-acting antiviral regimens developed thus far.
We discussed:
· The global distribution and prevalence of GT-3, which many may be surprised to learn is the second most prevalent genotype globally after genotype 1
· Issues around access, and restrictions to access, to HCV treatments, particularly in the context of the unique accelerated disease progression associated with GT-3
· Currently approved and available GT-3 treatment options
· The need for broader education for healthcare providers, health officials and patients to better understand the uniqueness of GT-3
· The need for additional data and information regarding unique patient populations who may be more affected by GT-3. For example, it is suggested that patients with a history of injection drug use may be more likely to have GT-3, but this may also depend on their ethnic and geographical background. A patient born in India where GT-3 is prevalent, who may have a history of intravenous drug use (IDU), but who resides in the USA or Western Europe may be more likely to have GT-3 compared with individuals from other ethnic or geographic backgrounds
Regarding the last point, it is important for such a person to undergo screening and for the patient and their healthcare provider to understand that this particular genotype of HCV infection might progress more quickly compared with other genotypes, and that earlier treatment may be required.
Finally, health officials, guidelines committees and, perhaps most importantly, insurance payers need to appreciate the issues surrounding HCV GT-3 and to reflect these in clinical guidelines, and not to restrict access to treatment for this group of patients.
The objectives of the meeting were to:
· Discuss evolving clinical practice and guidelines for treating HCV GT-3 patients
· Assess if and when HCV GT-3 patients should be considered and prioritized for therapy
· Evaluate recent GT-3 clinical trial and real-world data
The discussion in the meeting centered around currently approved medications.
The following participated in the meeting:
Healthcare professionals
Professor Ira Jacobson (Co-Chair)
Professor Heiner Wedemeyer (Co-Chair)
Dr. Alessio Aghemo
Dr. Kimberly Brown
Dr. Hugo Cheinquer
Professor Jan Gerstoft
Professor Christoph Hezode
Professor Manuel Romero-Gomez
Professor Christoph Sarrazin
Professor Ola Weiland*
Professor Jean-Pierre Bronowicki*
*Unable to attend due to last-minute conflict
Patient Advocacy Advisor
Jules Levin
Bristol-Myers Squibb (in person)
Dr Melissa Harris
Dr Szilvia Mosolits
David Richwine
Robert Perry
-------------------------------------------
The key takeaways agreed by the attendees:
· The unique pathophysiology and risk of progression of HCV GT-3 means infected patients should be considered an urgent priority for treatment
· Current European treatment guidelines reflect best practice in the management of GT-3 patients and may be used to guide treatment where the recommended regimens are available
· Both clinical trial and real-world data should be taken into account when deciding on the management of GT-3 patients
· Further urgent education of physicians regarding the importance of optimally effective and timely treatment of GT-3 patients is needed
· Prioritization of access to treatment for GT-3 patients should be ensured
-----------------------------------------------
Global Distribution and Prevalence of GT-3
GT-3 represents an important global burden. It is a unique genotype, with distinct challenges compared with genotype 1 and others. Although the unique aspects of GT-3 are not widely recognized by the community (clinicians, patients, health officials and insurers), there are several key points about its epidemiology, and disease characteristics, as well as the care and treatment of patients infected with this genotype, which are noteworthy.
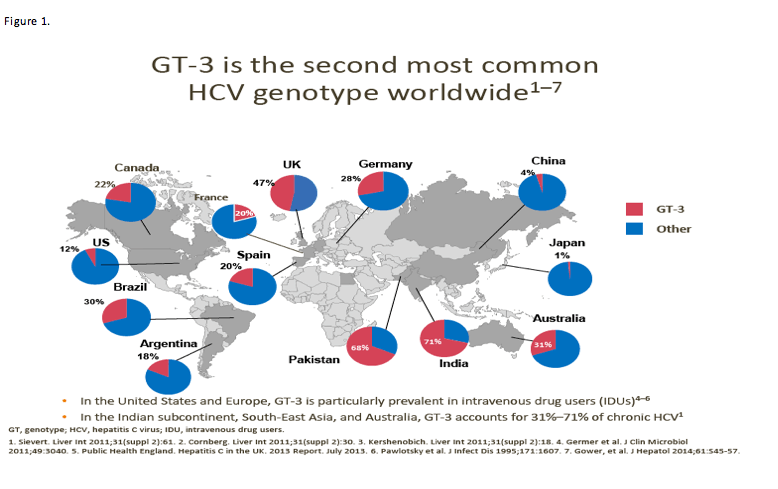
Many may find it surprising that GT-3 is the second most common genotype worldwide, and that its prevalence varies quite a lot across countries and continents.
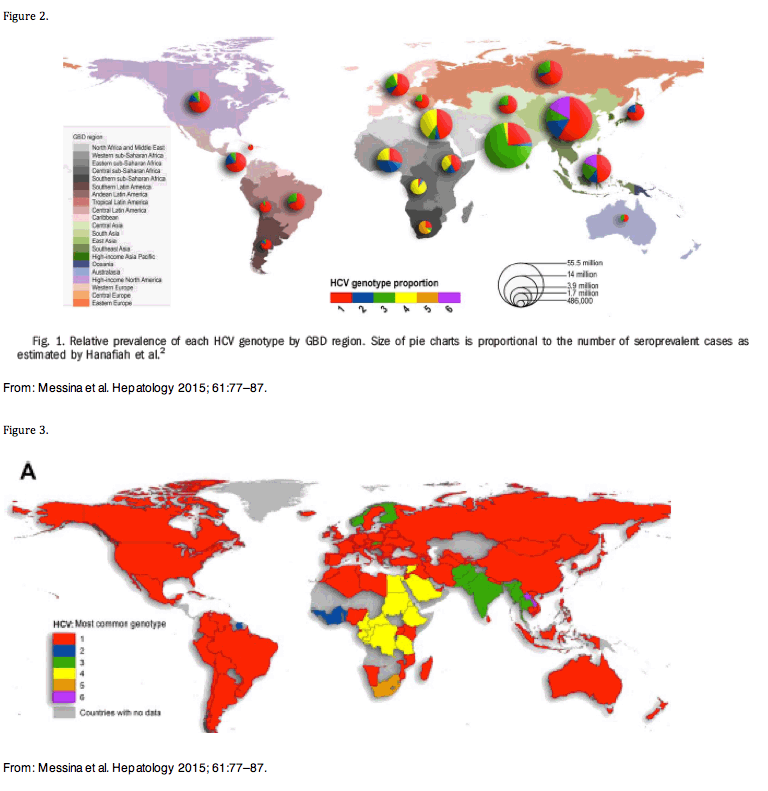
HCV GT-1 is the most prevalent HCV genotype worldwide, comprising 83.4 million cases (46.2% of all HCV cases), approximately one-third of which are in East Asia. [Messina et al. Hepatology 2015; 61:77-87.] GT-3 is the next most prevalent globally (54.3 million, 30.1%). [Messina et al. Hepatology 2015; 61:77-87.]
In the USA, estimates of GT-3 distribution generally range from 10% to 15% across all 50 states, with some having a prevalence as low as 4% and some as high as 18%. [LabCorp - http://www.natap.org/2015/HCV/061515_05.htm]
Data in Figure 1 suggest that GT-3 prevalence among HCV patients is up to 47% in the UK, 30% in Brazil, 20% in Spain and 20% in France.
During the meeting it was agreed by the meeting participants that it is important to update the prevalence data for GT-3. It would also be useful to understand a better breakdown of who has GT-3 - that is, what is the prevalence among IDUs and as well among various ethnic populations, as many patients in the US, as well globally, are not aware of the importance of treating this genotype.
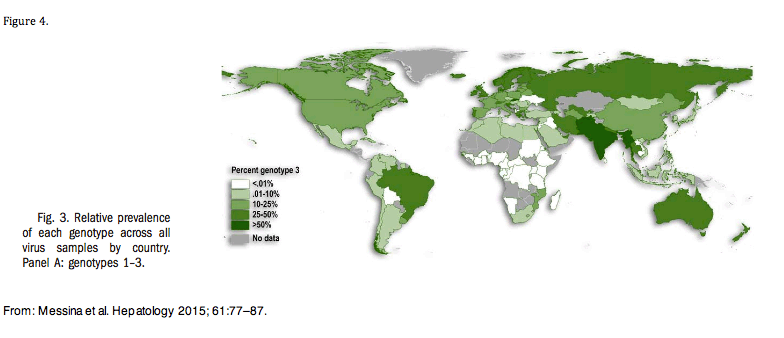
Figure 3 provides a feeling of the relative prevalence of GT-3 across the globe.
----------------------------
Genotype 3: Unique increased risk for disease progression
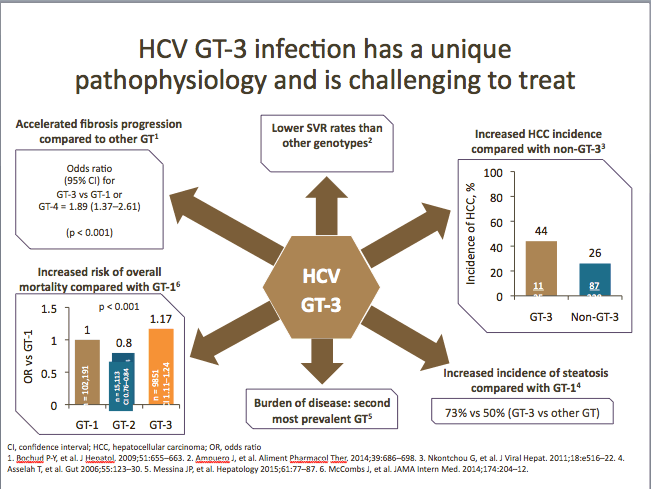
HCV GT-3 infection has a more progressive pathophysiology (accelerated liver fibrosis progression, increased risk of mortality, increased incidence of steatosis and HCC, and lower SVR rates [with IFN-free regimens]) vs other genotypes, and is recognized by many physicians in the majority of countries as an urgent priority for treatment.
In a retrospective study, carried out in France, of a large cohort of patients with HCV cirrhosis and persistent viral replication who were prospectively screened for HCC, it was observed that infection with GT-3 was associated with an increased risk for HCC vs non-GT-3 groups in univariate and multivariate analyses, taking into account the most commonly recognized risk factors. These finding reinforce the need to achieve an SVR... Furthermore, in a multivariate analysis, GT-3 was independently associated with an increased risk of HCC (hazard ratio [HR] 3.54 [95% confidence interval {CI} 1.84-6.81], p = 0.0002), even after adjustment for prothrombin activity and alcohol abuse (3.58 [1.80-7.13]; p = 0.003). Thus, for patients with HCV cirrhosis and ongoing HCV infection, infection with GT-3 is independently associated with an increased risk of HCC development. [G. Nkontchou, Journal of Viral Hepatitis, Oct 2011] [http://www.natap.org/2015/HCV/061515_07.htm].
In another study of US Veterans with HCV, it was observed that despite being younger, patients with GT-3 had a higher risk of developing cirrhosis (unadjusted HR 1.40 [95% CI 1.32-1.50]) and HCC (unadjusted HR 1.66 [95% CI 1.48-1.85]) than HCV GT-1 patients. After adjustment for pre-specified demographic, clinical and antiviral treatment factors, the risk of cirrhosis and HCC was 31% (adjusted HR 1.31 [95% CI 1.22-1.39]) and 80% (adjusted HR 1.80 [95% CI 1.61-2.03) higher in patients with GT-3 compared with GT-1 infected patients. [Fasiha Kanwal, Hepatology July 2014] [http://www.natap.org/2014/HCV/071614_03.htm]
Physicians at our meeting from France, Brazil and the US treat GT-3 patients irrespective of fibrosis status; however, it was agreed there is a need for education surrounding the urgency to treat GT-3 patients. In contrast, in Italy and Denmark, GT-3 is not considered a priority in current practice; in Italy, only patients ≥ F3 fibrosis score, extra-hepatic manifestations or organ transplant (heart and lung, but not hepatic) are eligible to receive treatment.
Treatment Guidelines and Best Practice
The meeting participants/advisors agreed that the EASL Guidelines [2015, http://www.natap.org/2015/EASL/EASL_02.htm], available at the time of the meeting, were the most up-to-date recommendations regarding GT-3. Access to treatment for GT-3 patients is a special problem that needs attention, and continued awareness regarding this is important.
Although some knowledgeable HCPs are familiar with GT-3 and the need to consider early and optimally effective treatment, it was considered at the meeting that there is a need to educate a wider community of clinicians, health officials and patients regarding the unique aspects of GT-3 (including its accelerated disease progression, the need to treat sooner rather than later, and the available treatment options).
Treatment must be undertaken in a considered manner, as HCV GT-3 is more difficult to treat than GT-1. Therefore, it is important to understand the data from the clinical studies, including SVR rates, and concerns regarding NS5A resistance. Patients with any genotype who fail therapy that includes an NS5A inhibitor may develop an NS5A-resistant variant, which may affect the effectiveness of future therapy that includes an NS5A inhibitor. In addition, data demonstrates that pre-existing NS5A resistance mutations may affect the ability to achieve SVR, increasing the risk of patients not achieving an SVR when treated.
A number of barriers to the treatment of GT-3 patients were discussed at the meeting. Because GT-3 is a difficult-to-treat genotype, some GT-3 patients may have been 'warehoused' in order to wait for the approval of treatment regimens that may offer more effective outcomes. In addition, access to treatment is a crucial barrier; with restricted access to treatment for IDUs and for patients with early HCV disease, creating a problem for GT-3 patients. GT-3 patients at an early disease stage still face the prospect of accelerated disease progression, and unless stakeholders and payers are educated about the need to treat GT-3 infection, patients can be at increased risk of the complications of their disease.
Advisors' comments
The advisors agreed that the recent EASL recommendations for treatment of patients infected with HCV are forward looking, and already influence practice in several countries, including non-EU ones (e.g. Brazil and Australia).
However, these are only clinical recommendations, and cost-efficacy is an important driver of treatment in many countries
Although in many countries patients infected with GT-3 are treated, reimbursement is often provided only for those with fibrosis stage ≥ F3. However, some countries (e.g. France) provide reimbursement for all GT-3 patients, regardless of staging, and clinicians in other countries (e.g. Brazil) are discussing a similar approach with payers.
Most countries have national treatment recommendations; however, these show great variability, reflecting the uncertainties that still exist regarding best practice (e.g. use of pegylated [peg]IFN-based regimens vs direct-acting antiviral [DAA]-based regimens, duration of treatment and use of ribavirin [RBV]).
The advisors agreed:
· There is a need for consensus regarding optimal patient management
· There is a need for improved awareness among physicians and payers regarding urgency to treat and optimally effective treatment regimens
· There is a need to increase knowledge regarding the utility of available therapies for GT-3 patients
Although thought leaders are aware of the latest data regarding GT-3 and available treatment options, many physicians in the wider medical community are not, and wider communication of this information is therefore necessary. In addition, from a patient perspective, education on the nuances of therapy would be useful, particularly about HCV GT-3.
Further data regarding the prevalence of GT-3 in various patient subpopulations (marginalized patient populations) would be useful and would allow tailored education programs for these groups regarding HCV. Such groups are not as easy to educate as HIV patients, and adherence in HCV patients is generally lower than in HIV patients, possibly because there is more acceptance of complicated dosing regimens by HIV patients.
In the US, where DCV was approved in July 2015, there may have been a rationale for warehousing GT-3 patients (in contrast to previous best practice); this applies to non-cirrhotic patients (who are in a position of being able to wait for treatment) as well as pre- and post-liver transplant patients.
Access to treatment is important, and discussion with payers is needed to ensure that the regimens optimal for efficacy and safety are included in payer recommendations.
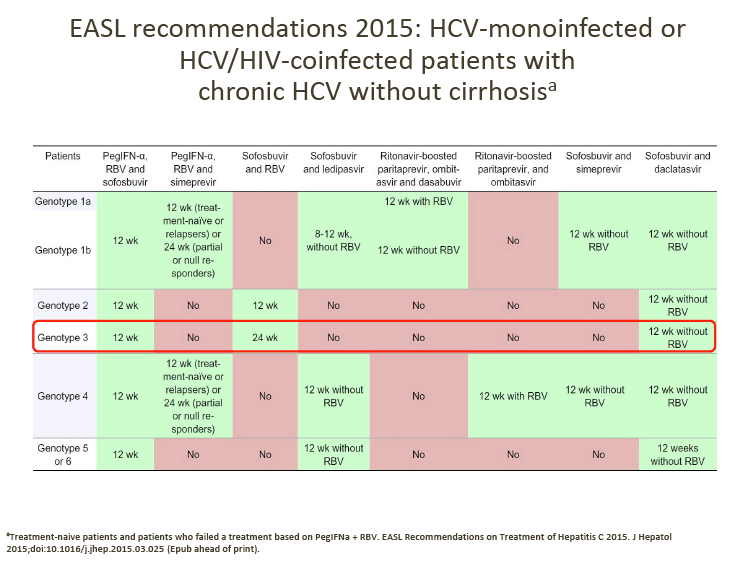
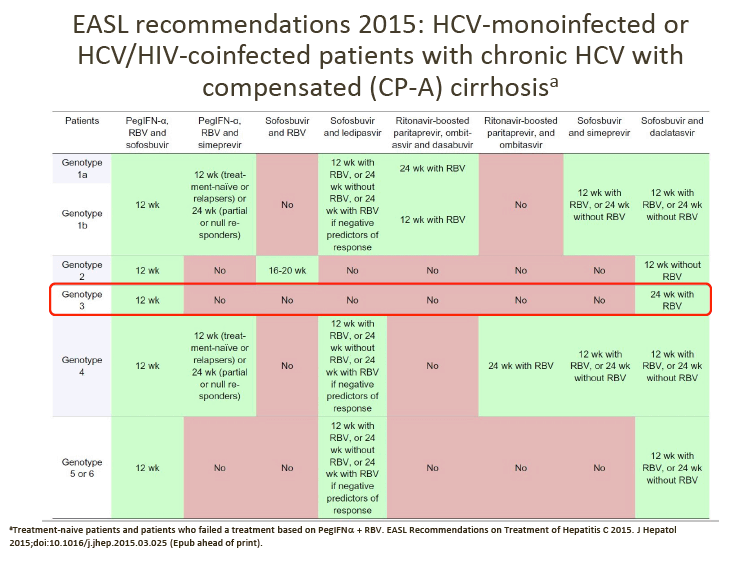
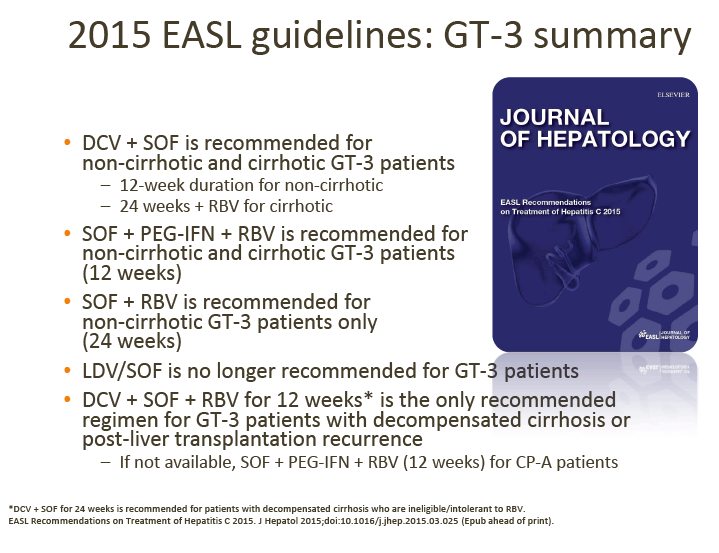
GT-3 Treatment
This section presents the study results for currently approved medicines for GT-3. Advisors at the meeting considered the up-to-date study results for these treatments.
There are several currently approved regimens that can be used for GT-3 treatment as outlined in the 2015 EASL recommendations above.
At the June GT-3 meeting, advisors agreed the following points.
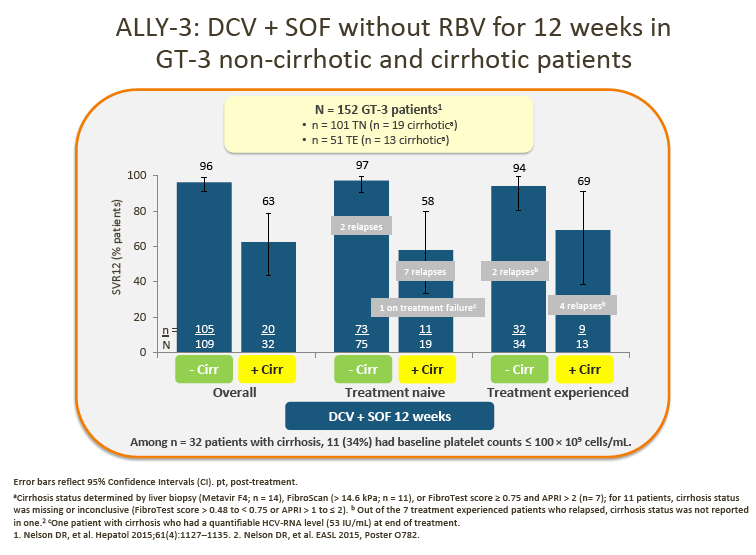
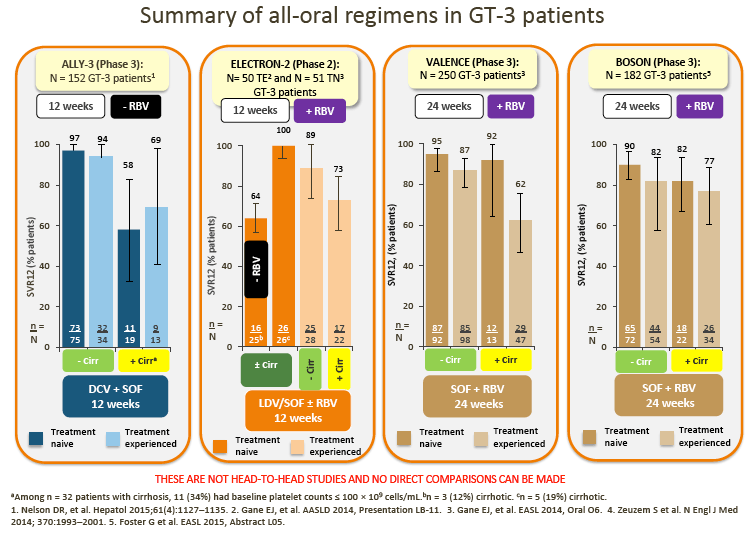
1. Daclatasvir (DCV) + sofosbuvir (SOF) is their preferred choice of treatment for GT-3 patients, although treatment duration (12 vs 24 weeks) and the addition of RBV varies between countries, depending on the patient's cirrhosis status.
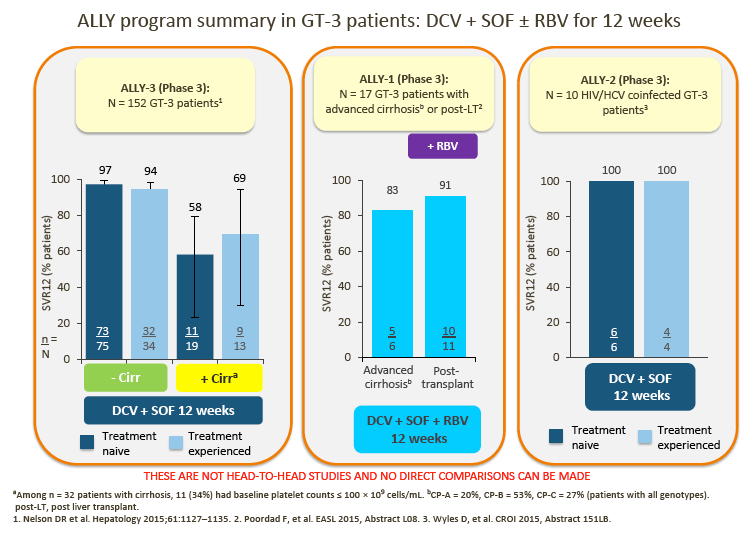
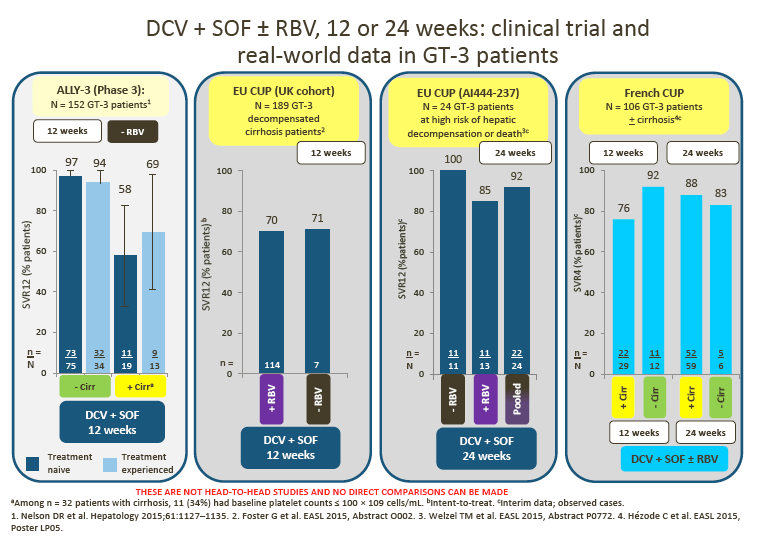
2. Data from the ALLY-3 study show high SVR rates (97% in treatment-naive [TN] patients and 94% in treatment-experienced [TE] patients) in non-cirrhotic patients following treatment with DCV + SOF (RBV-free) for 12 weeks.
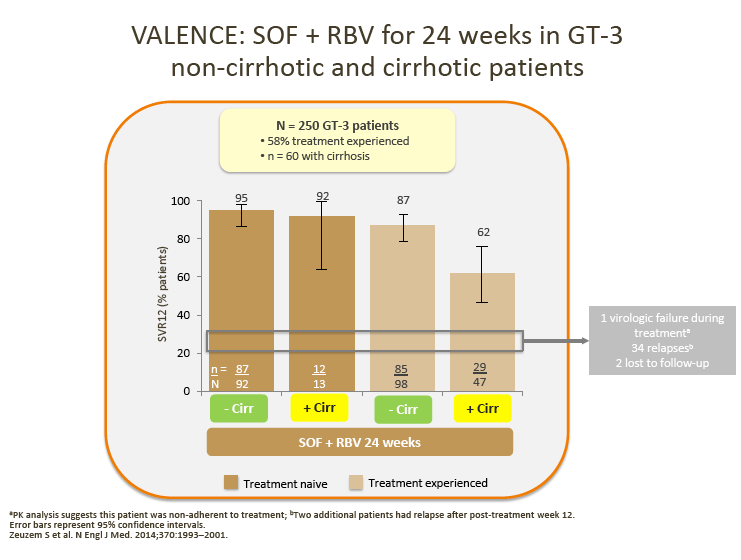
· Although no direct comparison is possible, ALLY-3 SVRs with DCV + SOF appear numerically higher compared with those observed in non-cirrhotic patients treated with SOF + RBV for 24 weeks in the VALENCE or BOSON studies
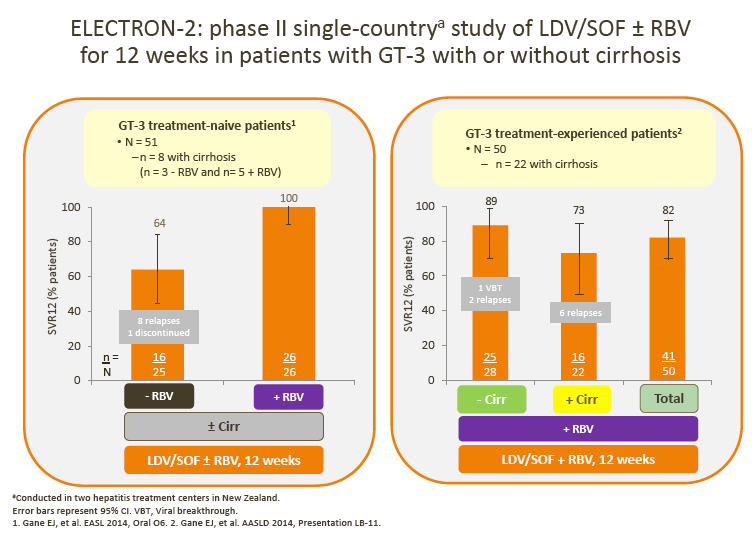
· SVR rates in ELECTRON-2 appear numerically lower in the RBV-free arm (64%) following treatment with ledipasvir (LDV)/SOF; this observation is in line with the > 1000 times lower in vitro potency of LDV vs DCV against GT-3 replicons (as discussed by Dr Brown)
3. In addition, the increased efficacy of LDV/SOF with RBV adds to the discussion regarding the potential role of RBV as an adjunct in several DAA regimens (Gane et al, ELECTRON 2)
· In ALLY-3, lower SVR rates were observed in cirrhotic than in noncirrhotic patients (63% overall for TN/TE patients combined).
· The advisors agreed that extending treatment to 24 weeks with or without RBV for cirrhotic patients may be necessary in order to maximize efficacy
· Overall, the consensus between the advisors was that they are willing to overtreat certain patients (e.g. those with cirrhosis) until more data are available to inform their decisions. From a patient perspective, it was also noted that it is important not to compromise the efficacy of treatment. In addition, predictors of response would be useful in order to optimize regimens used to treat patients with cirrhosis
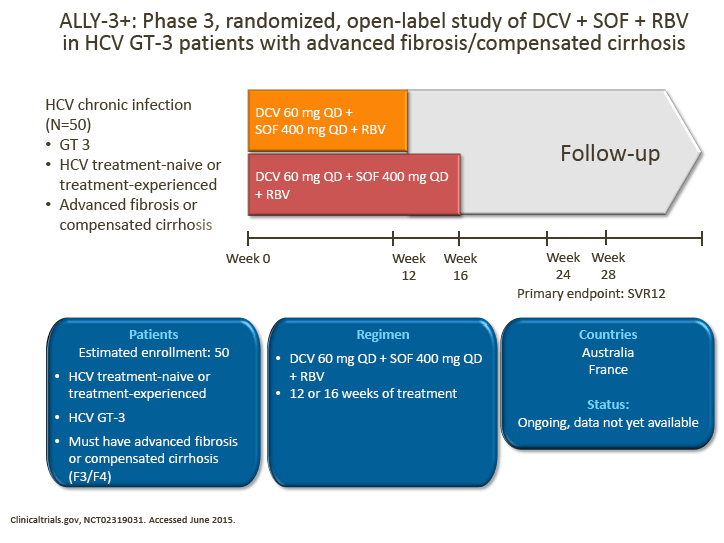
· It was agreed that the ALLY-3+ study of DCV + SOF + RBV for 12 or 16 weeks in patients with advanced fibrosis/compensated cirrhosis will provide further information regarding the utility of RBV and treatment duration up to 16 weeks in these patients
· These additional data will be informative, although given the study population size and the absence of a 24-week arm, it may not provide a definitive answer for how to treat patients with compensated cirrhosis
· The advisors suggested that exploring a 24-week treatment duration may provide additional insights in these patients
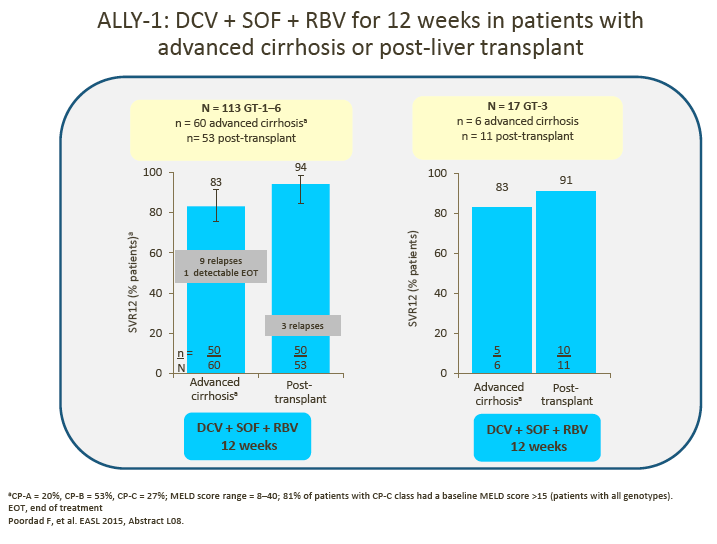
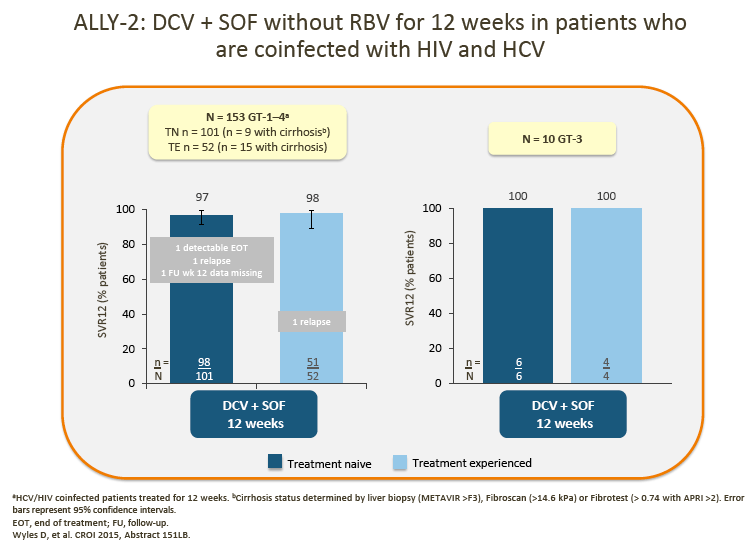
4. The advisors were encouraged by the data presented for the small number of GT-3 patients in ALLY-1 and ALLY-2.
· 12-week DCV + SOF showed encouraging SVR rates in HIV/HCV coinfected patients and, with the addition of RBV, in patients with advanced cirrhosis and post-liver transplant
5. Advisors suggested that further information regarding baseline NS5A RAVs (A30 and Y93) and their influence on DCV response, particularly in cirrhotic patients, would be useful.
· It was suggested that this may be achieved through either sub-analyses of existing study data (e.g. ALLY-3 or real-world studies) or new studies
· Additionally, it was suggested that baseline resistance testing of patients may also help nuance treatment, particularly in patients with cirrhosis
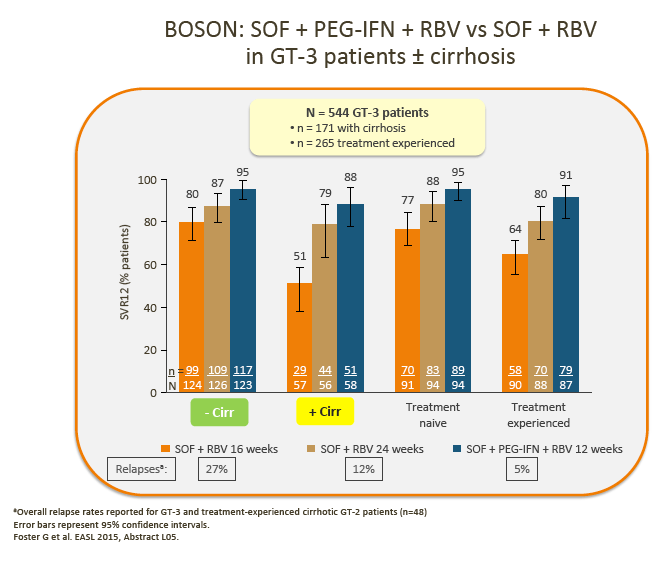
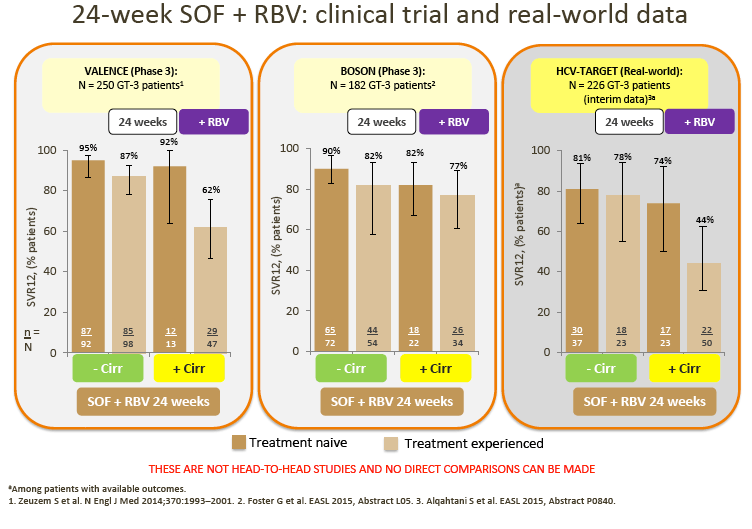
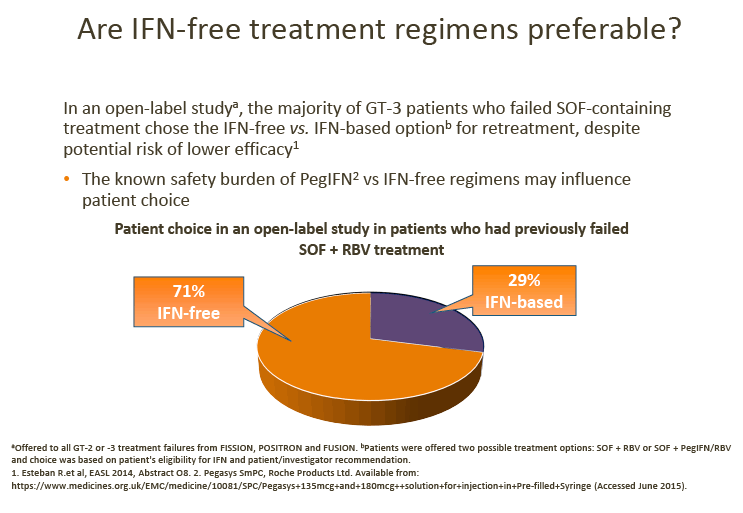
6. Although pegIFN + SOF + RBV may be used in some countries (France and the US), and despite the recent BOSON data demonstrating improved SVR with 24 weeks of treatment with PegIFN + SOF + RBV vs 24 weeks of treatment with SOF + RBV, uptake of this regimen is generally low, and it is not considered a realistic option in most countries.
· Advisors considered that the low uptake of IFN-based treatments may be a result of patient preference and concerns regarding the IFN side-effect profile
· Some countries, e.g. Denmark and Spain, do not use IFN-based regimens
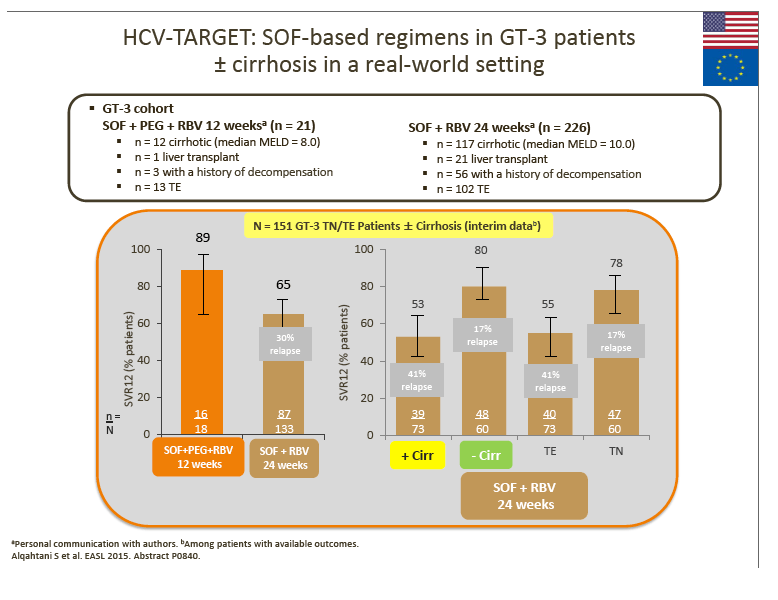
7. The advisors considered that the real-world data from the HCV-TARGET study with 24-week SOF + RBV suggest that this regimen may not be optimal for GT-3 patients.
· Although not directly comparable, SVR rates appear lower (especially in TE and cirrhotic patients) vs SOF + RBV-based clinical trial and other real-world data (with DCV + SOF ± RBV). SOF + RBV (24 weeks) or pegIFN + SOF + RBV (12 weeks) was used in the US prior to the recent approval of DCV
8. Advisors agreed that real-world data support the use of DCV + SOF ± RBV (12 or 24 weeks) for the treatment of GT-3 patients.
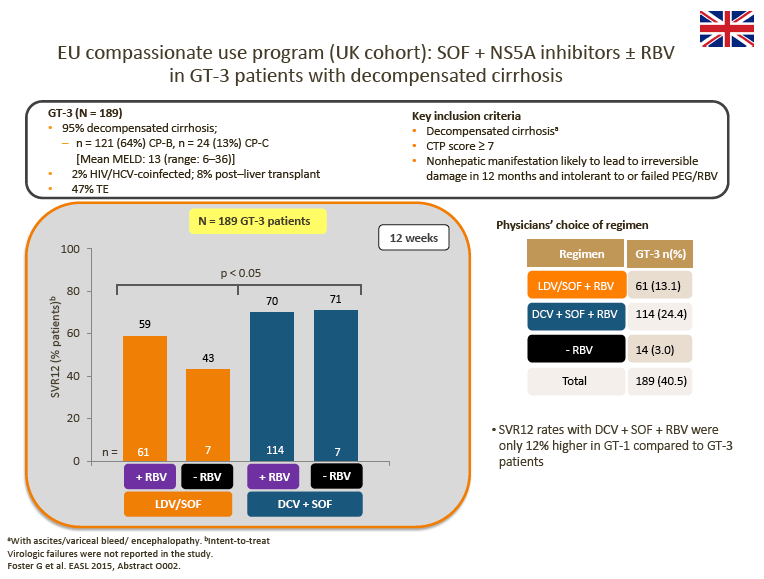
· Advisors were impressed by the data from the UK cohort of the EU Compassionate Use Program (CUP), which showed superior SVR rates for 12-week DCV + SOF ± RBV compared with LDV/SOF ± RBV in patients with decompensated cirrhosis
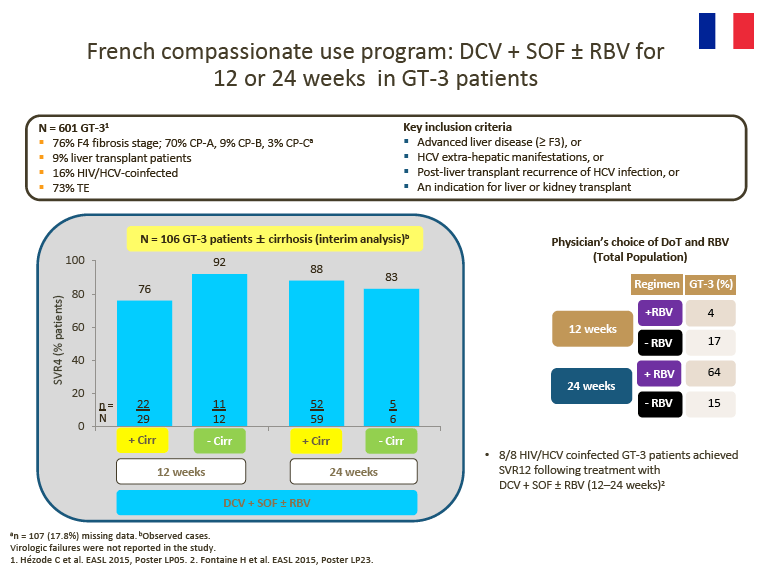
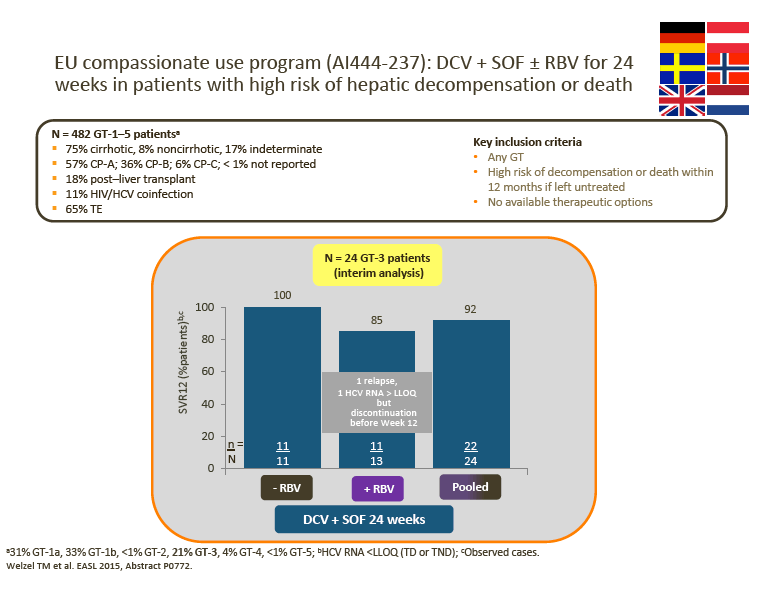
Interim DCV + SOF ± RBV data from the EU CUP (AI444-237) and the French CUP show consistently high observed SVR rates in patients with advanced liver disease
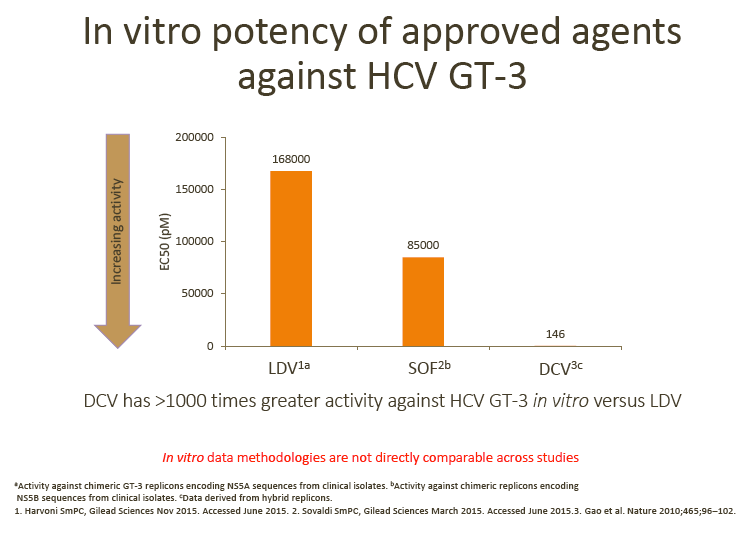
|
|
| |
| |
|
|
|