| |
HCV Guidelines - Genotype 1b.....Abbvie Granted
Orphan Drug Designation for pedisatric patients
|
| |
| |
AbbVie announced that the FDA granted VIEKIRA orphan drug designation for the investigational treatment of HCV infection in pediatric patients, 0 - 16 years of age. Orphan drug designations recognize the need for a treatment of a rare disease or condition. AbbVie will conduct an investigational clinical study to evaluate the efficacy and safety of VIEKIRA with or without RBV in pediatric patients with chronic HCV infection. VIEKIRA is currently not approved for this indication. July 24 2015
HCVGuidelines
http://www.hcvguidelines.org/full-report/initial-treatment-hcv-infection
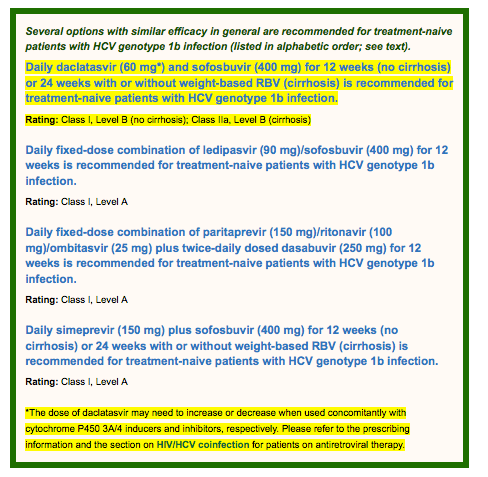
For HCV genotype 1b-infected, treatment-naive patients, there are 4 regimens of comparable efficacy, as outlined above.
There is no measurable difference demonstrated to date in treatment response to daclatasvir and sofosbuvir or ledipasvir/sofosbuvir for HCV genotype 1 subtypes, thus the supporting evidence remains the same as for HCV genotype 1a-infected patients (see Genotype 1). In the ALLY-2 arm of daclatasvir and sofosbuvir for 12 weeks in treatment-naive patients, only 12 were genotype 1b and all achieved SVR12. (Wyles, 2015) Furthermore, in the ALLY-1 study all 11 genotype 1b infected patients with advanced cirrhosis achieved SVR12. Due to the limited numbers of genotype 1b patients represented in the phase 3 trials of this regimen, there is not enough evidence to support a different approach by subtype at this time.
PrOD (plus RBV for those with cirrhosis) was approved by the FDA for the treatment of HCV genotype 1b infection in treatment-naive patients based on 3 registration trials: SAPPHIRE-I (151 treatment-naive patients with HCV genotype 1b and without cirrhosis), PEARL-III (419 treatment-naive patients, all with genotype 1b and without cirrhosis), and TURQUOISE-II (119 treatment-naive and -experienced patients with genotype 1b with cirrhosis). SAPPHIRE-I reported a high SVR12 rate (98%) with 12 weeks of PrOD and RBV in patients with HCV genotype 1b. (Feld, 2014) Given the high SVR12 rates seen in SAPPHIRE-I, PEARL-III was specifically designed to determine the role of weight-based RBV with PrOD in treatment-naive patients with HCV genotype 1b without cirrhosis. (Ferenci, 2014) SVR12 rate was 99% in both arms, confirming that there is no added benefit from the use of weight-based RBV for patients without cirrhosis who have HCV genotype 1b infection. TURQUOISE-II enrolled treatment-naive and -experienced patients with CTP class A cirrhosis to receive either 12 weeks or 24 weeks of treatment with PrOD and RBV. Overall, SVR12 rates were 98.5% in the 12-week arm and 100% in the 24-week arm. (Poordad, 2014) To address the need for RBV with this regimen in patients with HCV genotype 1b and cirrhosis, the TURQUOISE-III study evaluated the safety and efficacy of PrOD without RBV for 12 weeks in patients with HCV genotype 1b infection and compensated cirrhosis. Sixty patients (62% men, 55% treatment experienced, 83% with the IL28B non-CC genotype, 22% with platelet counts <90 x 109/L, and 17% with albumin levels <3.5 g/dL) were enrolled. All patients completed treatment, and all patients achieved an SVR12. On the basis of this study, treating patients with HCV genotype 1b with PrOD but without RBV is recommended, regardless of prior treatment experience or presence of cirrhosis. (Feld, 2015)
To date, there is no measurable difference demonstrated in treatment response to simeprevir plus sofosbuvir for HCV genotype 1 subtypes (with the exception of patients with genotype 1a with cirrhosis who also have the baseline Q80K mutation), thus the supporting evidence remains the same as for HCV genotype 1a-infected patients (see Genotype 1).
The safety profiles to date of all recommended regimens above are excellent. Across numerous phase III programs, less than 1% of patients without cirrhosis discontinued treatment early and adverse events were mild. Most adverse events occurred in RBV-containing arms. Discontinuation rates were higher for patients with cirrhosis (approximately 2% for some trials) but still very low.
|
|
| |
| |
|
|
|