| |
Sofosbuvir plus Simeprevir Treatment of Recurrent
Genotype 1 Hepatitis C after Liver Transplant
|
| |
| |
Download the PDF here
Clinical Transplantation Sept 2015
Abstract
Patients with recurrent hepatitis C (HCV) infection post liver-transplant can be difficult to treat safely and effectively. A prior (COSMOS) study in non-transplant HCV patients, using sofosbuvir plus simeprevir, had high efficacy and tolerability in treating HCV genotype 1 patients, even prior non-responders to interferon therapy and those with cirrhosis. Our aim was to evaluate the efficacy of sofosbuvir and simeprevir in genotype 1 HCV post-liver transplant patients.
In this prospective, observational study, patients received sofosbuvir 400mg plus simeprevir 150mg daily for 12 weeks without ribavirin. The primary endpoint was a sustained virologic response 12 weeks after the end of therapy.
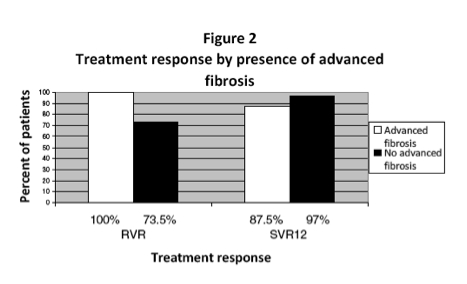
Forty-two patients completed treatment. Twenty-six percent started treatment <6 months post-liver transplant. Nineteen percent of the included patients had cirrhosis, 14% with decompensation. At week 4 on treatment, 21% of patients had detectable virus but at the end of treatment, 100% were undetectable. Twelve weeks after the end of treatment, 95% of patients had undetectable hepatitis C. The regimen was generally well tolerated.
Conclusion
The oral regimen of sofosbuvir plus simeprevir without ribavirin is efficacious and well tolerated in the treatment of genotype 1 hepatitis C patients post-liver transplant.
|
|
| |
| |
|
|
|