| |
On the origin of liver regeneration
|
| |
| |
Download the PDF here
Download the PDF here
Download the PDF here
Nature Reviews Gastroenterology & Hepatology
01 September 2015
Katrina Ray
A remarkable feature of the liver is that it can regenerate. This property has been under intense scrutiny as studies have tried to determine the cellular source of liver regeneration. Now, unique populations of cells with the capacity to repopulate the liver have been identified in two new studies. The researchers used similar approaches to identify these cells, but examined the issue in different scenarios-homeostasis and chronic liver injury.
In the first study, published in Nature, a unique population of proliferating and self-renewing hepatocytes was identified. These cells were present in a specific niche next to the central vein in the liver lobule, and contributed to liver homeostasis.
As Wnt signalling is crucial to maintain many tissue stem cells, Wang et al. performed genetic lineage tracing of Axin2+ cells (a marker of Wnt-responsive cells) in the livers of mice, finding Axin2+ cells located around the central vein. Following the fate of these cells revealed that the Axin2+ pericentral cells generate clones of hepatocytes that expand over time from the central vein to the portal vein. After 1 year, on average, descendants of Axin2+ cells replaced ~30% of the entire liver area, accounting for ~40% of hepatocytes.
Further characterization of these Axin2+ pericentral hepatocytes revealed that they self-renew (a defining property of stem cells), express Tbx3 (an early liver progenitor marker) and are mostly diploid. By contrast, the descendants of these Axin2+ cells mature into polyploid cells after leaving the pericentral niche and no longer express Tbx3. Finally, central vein endothelium was confirmed as a Wnt-producing niche and local source of Wnt signals required for pericentral cell proliferation.
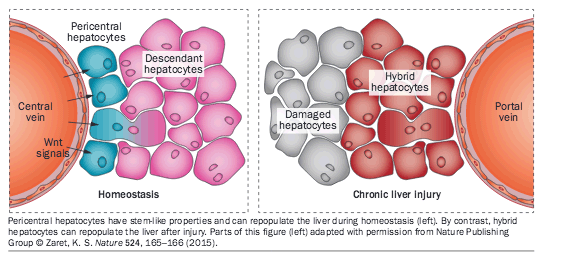
Pericentral hepatocytes have stem-like properties and can repopulate the liver during homeostasis (left). By contrast, hybrid hepatocytes can repopulate the liver after injury. Parts of this figure (left) adapted with permission from Nature Publishing Group Zaret, K. S. Nature 524, 165-166 (2015).
"Our findings fundamentally change the way we think about the basic cell biology of hepatocytes," says first author Bruce Wang. "It means that the liver ... is maintained during homeostasis by a stem cell population," he adds, "and that hepatocytes are not equivalent in their proliferative potential, suggesting that hepatocytes are not a single cell type."
In the second study, published in Cell, researchers identified a subpopulation of liver cells in the periportal area, so-called hybrid hepatocytes. These cells did not expand during homeostasis, but proliferated extensively after chronic liver injury, replenishing the liver without giving rise to hepatocellular carcinoma, an important point given that compensatory hepatocyte proliferation has a key role in liver carcinogenesis.
In mice, Font-Burgada et al. observed hybrid hepatocytes in the periportal liver area only (comprising 4.53% of all hepatocytes present). These cells had high regenerative capacity and expressed normal levels of Hnf4α but low levels of Sox9 (mixed markers of hepatocytes and bile duct cells) with a unique transcriptome.
Tracking these cells over time demonstrated that hybrid hepatocytes proliferate (extending from the portal vein to the central vein) and repair the liver after damage in several models of chronic liver injury, with the capacity to transdifferentiate into ductal cells after cholestatic liver injury. Moreover, hybrid hepatocytes could be transplanted into mice with severe liver damage, where they clonally expanded across all liver lobules and promoted survival (none of these mice died, whilst 90% of nontransplanted mice and >50% mice transplanted with conventional hepatocytes died).
"Our model of liver regeneration based on hybrid hepatocytes changes substantially the way we have understood how liver regenerates after damage," says first author Joan Font-Burgada. "It was very striking to see how much tissue was generated from this population of cells."
"What is amazing is that you would think a biological problem as to which way hepatocytes migrate (if at all) would be simple to solve, but it has proved problematic," says Malcolm Alison (Queen Mary University of London, UK), who was not involved in the new studies. "To me, these new data [from Wang et al.] are really surprising," says Alison adding that some previous evidence from animal and human studies showed that liver cells migrate away from the portal areas to the central vein, as was observed in the Font-Burgada et al. study. "Maybe the liver is adaptable having alternative stem cell niches, one acting as a back-up if the other is damaged," he considers.
References
1Wang, B. et al. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 524, 180-185 (2015)
2Font-Burgada, J. et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162, 766-779 (2015)
----------------------------
Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer
Summary
Compensatory proliferation triggered by hepatocyte loss is required for liver regeneration and maintenance but also promotes development of hepatocellular carcinoma (HCC). Despite extensive investigation, the cells responsible for hepatocyte restoration or HCC development remain poorly characterized. We used genetic lineage tracing to identify cells responsible for hepatocyte replenishment following chronic liver injury and queried their roles in three distinct HCC models. We found that a pre-existing population of periportal hepatocytes, located in the portal triads of healthy livers and expressing low amounts of Sox9 and other bile-duct-enriched genes, undergo extensive proliferation and replenish liver mass after chronic hepatocyte-depleting injuries. Despite their high regenerative potential, these so-called hybrid hepatocytes do not give rise to HCC in chronically injured livers and thus represent a unique way to restore tissue function and avoid tumorigenesis. This specialized set of pre-existing differentiated cells may be highly suitable for cell-based therapy of chronic hepatocyte-depleting disorders.
-------------------
Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver
Discussion
In this paper we present a new view of hepatocyte homeostasis in the uninjured liver (Fig. 6h). We have identified a Wnt-responsive cell population that resides within a confined niche around the central vein. These cells self-renew and contribute to hepatocyte maintenance by differentiating into and replacing other hepatocytes along the hepatic lobule in the normal liver. The existence of this pericentral cell population suggests that the fundamental mechanisms regulating liver renewal are similar to other organs in which homeostatic renewal involves small populations of stem cells that maintain the tissue. In the liver however, our model is novel because it was previously thought that all hepatocytes are equivalent in their renewal potential. In contrast, we show that hepatocytes are made up of more than one cell type and are not equivalent in replicative ability during homeostasis. Given the properties of the cell population under study, we postulate that the Wnt-responsive pericentral cells are hepatocyte stem cells.
Several features make pericentral cells unique compared to other hepatocytes. Although pericentral cells express markers common to other hepatocytes, they also specifically express Axin2, Tbx3 and GS while lacking CPS. Pericentral cells proliferate at a higher rate compared to other hepatocytes, an observation that is consistent with ref. 30. Furthermore, pericentral cells possess a diploid genome, in contrast to most other hepatocytes, which are polyploid. Finally, and most importantly, while pericentral Axin2+ cells can differentiate into all hepatocytes along the lobule, including those that line the portal vein, Axin2- hepatocytes do not replace pericentral cells during homeostasis. As pericentral cells can self-renew over the long term and differentiate into other hepatocytes, we suggest that they fit the functional definition of a stem cell.
The diploid nature of pericentral cells is important and surprising, although nuclear size measurements in rat livers have suggested the presence of smaller nuclei near the central vein31. This sheds light on a long-standing question in liver biology. Mature polyploid hepatocytes display chromosomal abnormalities5, 32 and display impaired replication5, 6. By maintaining a diploid genome, the pericentral cells would, like stem cells25, retain unlimited replicative potential. It is interesting to note that during the cell cycle, levels of Wnt signalling peak at the G2/M phase33. If Wnt proteins regulate expression of mitotic control genes such as the phosphatase Cdc2534, they could direct cells to mitosis and continued diploidy rather than to non-mitotic DNA replication and polyploidy.
A defining feature of pericentral cells is their localization to a Wnt-rich anatomical niche. While Wnt-regulated genes such as ß-catenin and Apc are known to function in liver development35 and zonation14, the types and sources of Wnt have not been identified. We found that Wnt9b is specifically expressed in endothelial cells at the central vein, adjacent to the pericentral cells, while Wnt2 is expressed in both the sinusoidal and central vein endothelial cells. Notably, Wnt2 produced by sinusoidal endothelial cells is known to be important for hepatocyte regeneration after injury26. Similarly, in other stem cell niches, lipid-modified Wnt signals act as short-range cues, maintaining stem cells in the immediate vicinity of the niche but not outside11.
It has been suggested that there may be a periportal source of new hepatocytes under normal conditions36. Our lineage tracing studies do not exclude the possibility that other sources of hepatocytes exist during homeostasis since after one year the descendants of pericentral cells replace on average only 40% of hepatocytes within the liver. However, a portal-based population would be regulated differently, since we find no expression of Wnt9b by the portal vein endothelium.
Liver is known to regenerate efficiently after injuries such as partial hepatectomy or chemical insult. It has been reported that during regeneration after chemical damage, a Wnt-responsive population of cells near the portal vein can be labelled by the Lgr5 receptor gene15. These cells, unlike pericentral Axin2+ cells, do not express hepatocyte genes, but subsequently differentiate into bile duct epithelial cells and hepatocytes and thus could be similar to injury-induced oval cells3. Clearly, Lgr5+/oval cells are distinct from the cells we identify here, as pericentral cells maintain hepatocyte homeostasis in the uninjured liver while Lgr5+/oval cells have only been reported after injury.
The stem cell marker Tbx3 is expressed widely in early liver hepatoblasts and is important for hepatoblast proliferation and initiation of hepatocyte differentiation19, 37. Our findings that pericentral cells also express Tbx3 leads to the intriguing hypothesis that pericentral cells may represent the persistence of an embryonic hepatocyte progenitor population into a self-renewing cell population in the mature liver.
It is noteworthy that liver cancer is often characterized by loss-of-function mutations in negative regulators of the Wnt pathway, including Axin and APC38. In a mouse model of liver cancer caused by Met overexpression, liver tumours were found to arise exclusively from cells located at the central vein39, 40, suggesting that pericentral Axin2+ cells, normally controlled by a paracrine Wnt signal, are precursors to liver cancer. This would explain why liver tumours contain mostly diploid cells41, an observation that was earlier rationalized by polyploid hepatocytes becoming diploid after oncogenic transformation.
|
|
| |
| |
|
|
|