| |
Why We Should Be Willing to Pay for Hepatitis C Treatment......
|
| |
| |
Download the PDF here
http://www.natap.org/2015/HCV/073115_01.htm
Clinical Gastroenterology and Hepatology October 2015
"overall budget needed to treat HCV is not huge and is reasonable when compared with that of HIV.....Therefore, HCV treatment should be not be restricted only to patients in advanced fibrosis stages. We have an opportunity to eliminate hepatitis C by taking appropriate and timely steps. We as a society should be willing to pay for the current HCV therapies by providing additional resources and giving the attention to hepatitis C that it deserves.......The discounted lifetime cost of treating 1 person with HIV in the United States is $315,000 in 2014 US dollars.19 The corresponding cost of curing HCV with oral DAAs is $58,000--which is only 18% of the total HIV treatment cost. HIV antiretroviral treatment is cost effective in the United States,20 HCV treatment is cost saving."
The launch of oral direct-acting antivirals (DAAs) to treat chronic hepatitis C virus (HCV) infection represents a significant shift in the HCV treatment paradigm. With DAAs, the sustained virologic response (SVR) (ie, efficacy of treatment) has increased to more than 90%, treatment duration has decreased to as few as 8 weeks, and these regimens have no major side effects. Coupled with the updates in HCV screening guidelines, use of new DAAs could make HCV a rare disease in the next 20 years in the United States.1
However, the high price of DAAs is a barrier, and has drawn criticism from patients and payers.2, 3, 4 Challenged with a budget needed to treat all HCV patients, Medicaid has restricted these treatments in at least 30 US states to patients with advanced fibrosis stage.5 With more than a million patients needing HCV treatment in the next 3 to 5 years in the United States, the high price of DAAs could impact the budget of private payers and government. On the other hand, several recent studies have shown that these drugs provide a good value for the money. Furthermore, the price of DAAs has decreased since their first availability. For example, the average discounts on sofosbuvir-based regimens in 2015 have been 46%.6 As additional antiviral drugs become available in the near future, drug prices may decrease even further.
Here, we discuss the value of HCV treatment with oral DAAs considering new discounts, the importance of treating all HCV patients, and how HCV treatment costs and value compare with that of human immunodeficiency (HIV) treatment.
Value of Hepatitis C Virus Treatment
Recently published cost-effectiveness studies have shown that HCV regimens based on sofosbuvir, ledipasvir, and simeprevir are cost effective for most patients.7, 8, 9, 10, 11, 12 The incremental cost-effectiveness ratios (ICERs) of these regimens (when compared with the old standard of care) ranged from $10,000 to $284,000 per quality-adjusted life-year (QALY) depending on the patient's status with respect to treatment history, HCV genotype, and cirrhosis status. The average ICER for all HCV patients was $55,400 per QALY.7 The ICERs of treatment with older therapies based on first-generation protease inhibitors, boceprevir and telaprevir, were between $17,000 and $103,000 per QALY, depending on disease stage.13, 14, 15, 16, 17 The ICERs of peginterferon-ribavirin (in comparison with peginterferon) were between $26,000 and $64,000 per QALY. In general, the ICERs were higher in patients with early stages of liver fibrosis than in patients with advanced fibrosis. Collectively, these data show that throughout its history, compared with the previous standard, overall the "new" HCV treatment costs an additional approximately $50,000 to $100,000 for 1 additional QALY gained and the DAAs are no exception.
Hepatitis C Virus Treatment Now Is Cost Saving
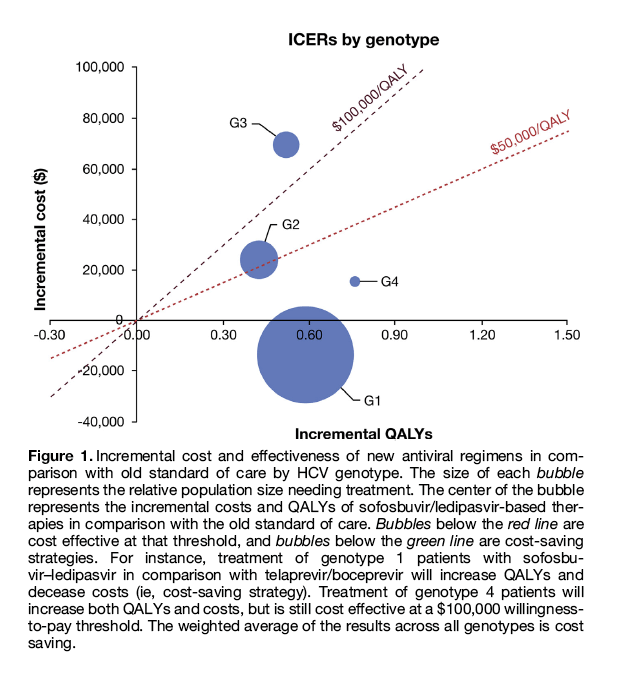
With recent rebates on drug prices, sofosbuvir-based treatment in 2015, on average, costs 54% of the wholesale acquisition cost.6 Applying these discounted drug prices to our previously published simulation model,7 we evaluated the cost effectiveness of DAAs. We found that compared with treatment with telaprevir/boceprevir or peginterferon-based therapies, treatment with sofosbuvir-ledipasvir regimens is cost saving in the majority of patients (ie, these regimens increased QALYs and saved health care costs) (Figure 1). This effect was most prominent in patients with genotype 1 infection. Treatment was not cost saving, although it was cost effective, in patients with other genotypes.
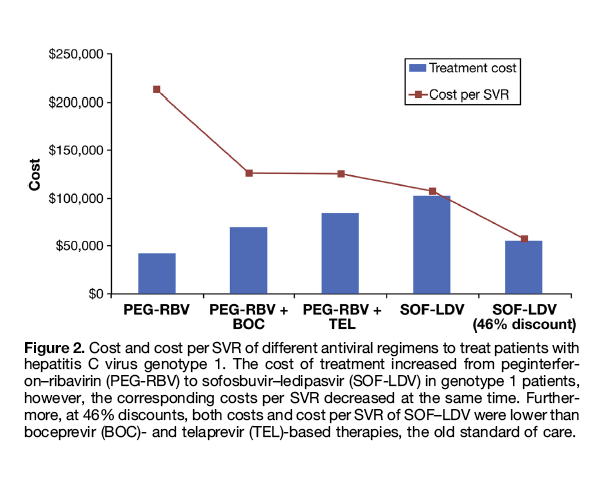
Decreased Cost per Sustained Virologic Response
Although the cost of antiviral treatment increased with the availability of new therapies, the cost per SVR has decreased. As shown in Figure 2, the cost of treating HCV genotype 1 with peginterferon-ribavirin, first-generation protease inhibitors, and sofosbuvir-ledipasvir (at wholesale acquisition cost) increased from $43,000 to $103,000 per patient. However, the corresponding costs per SVR decreased from $213,000 to $108,000. After applying the recent discounts (46%), the cost of treatment decreased to $56,000, which is less expensive than boceprevir- and telaprevir-based therapies, and the cost per SVR decreased to $58,000.
Health Economics of Hepatitis C Virus Versus Human Immunodeficiency Virus Treatment
HCV has superseded HIV as a cause of death in the United States since 2007.18 Therefore, to put the health economics of HCV into perspective, we can compare the cost of HCV treatment with DAAs with the cost of treating HIV. The discounted lifetime cost of treating 1 person with HIV in the United States is $315,000 in 2014 US dollars.19 The corresponding cost of curing HCV with oral DAAs is $58,000--which is only 18% of the total HIV treatment cost. HIV antiretroviral treatment is cost effective in the United States,20 HCV treatment is cost saving.
The total federal budget requested for HIV and acquired immune deficiency syndrome in 2015 was $24.2 billion, of which $17.5 billion was allocated to HIV treatment and care.21 Ryan White's Acquired Immune Deficiency Syndrome Drug Assistance Program, which provides access to HIV-related medications to people with HIV, was funded at $900 million. The federal spending on HCV treatment is unknown. However, using a simulation model, we predicted that the maximum 5-year budget needed to treat all patients (by private as well as government payers) who are candidates for HCV treatment would be $37 billion (ie, $7.4 billion per year).7 Of note, unlike HIV, HCV treatment offers a cure; therefore, annual spending on HCV treatment would decrease sharply in subsequent years.
Why We Should Be Willing to Pay for Hepatitis C Virus Treatment
The cost of HCV treatment with the available oral DAAs has decreased substantially since their first availability in 2014. Furthermore, we anticipate more discounts with increased competition from other manufacturers in the near future. The overall budget needed to treat HCV is not huge and is reasonable when compared with that of HIV. Therefore, HCV treatment should be not be restricted only to patients in advanced fibrosis stages. We have an opportunity to eliminate hepatitis C by taking appropriate and timely steps. We as a society should be willing to pay for the current HCV therapies by providing additional resources and giving the attention to hepatitis C that it deserves.
Jagpreet Chhatwal, Institute for Technology Assessment, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
Qiushi Chen, H. Milton Stewart School of Industrial and Systems Engineering, Georgia Institute of Technology, Atlanta, Georgia
Fasiha Kanwal, Houston Veterans Affairs Health Services Research and Development Center of Excellence, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, Department of Medicine, Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas
|
|
| |
| |
|
|
|