| |
Cost-effectiveness of Early Treatment of Hepatitis C Virus Genotype 1 by Stage
of Liver Fibrosis in a US Treatment-Naive Population - '$58 Bill to treat all'
|
| |
| |
Download the PDF here
Download the PDF here
"This analysis suggests that treatment with new HCV drugs is cost-effective when started with any evidence of fibrosis (stage F1)."
"The new HCV interferon-free therapies offer potentially huge individual and societal benefits but at a large cost. Health plans and health systems concerned about costs frequently require evidence of advanced liver fibrosis before authorizing the new therapies.11- 14 We herein examined the health impact, cost, and cost-effectiveness of earlier treatment.
Although early treatment with sofosbuvir-ledipasvir is expensive, the net cost is substantially lower owing to savings in medical care and the likelihood of later treatment with a delayed treatment policy. Furthermore, we found substantial short- and long-term health gains. Thus, for sofosbuvir-ledipasvir treatment, treating patients at all fibrosis stages compared with waiting for advanced fibrosis is cost-effective (<$50 000 per QALY gained). A detailed analysis of timing of therapy by fibrosis stage shows that treating the disease at as early as stage F1 is cost-effective (ICERs of $50 000-$150 000 per QALY gained) and less than $50 000 per QALY gained when treatment is initiated at stage F2 vs stage F3. The ICER is lower when treatment is initiated at stage F3 compared with waiting for cirrhosis (stage F4). Results are similar for treatment with other new antiviral regimens.
If 50% of eligible patients with HCV genotype 1 (ie, 686 000 patients) are treated regardless of fibrosis stage during the next 5 years, the estimated unadjusted treatment costs are $53 billion. With the 46% reduction in drug prices discussed above, the cost decreases to $29 billion. Alternatively, if 50% of these patients are treated at stages F3 and F4 during the next 5 years, the costs are $30 billion with sofosbuvir-ledipasvir treatment and $16 billion with a 46% decrease in drug prices. Figure 4 depicts the savings in lifetime health care costs exclusive of drug costs gained by treating all stages compared with treating stages F3 and F4, which is $3.3 billion with sofosbuvir-ledipasvir treatment."
"For budgetary considerations, if only 50% of eligible patients with HCV genotype 1 were to be treated with sofosbuvir-ledipasvir during the next 5 years, the cost of drugs in the United States would be $53 billion at current prices. Many payers negotiate prices, as has been seen with exclusivity deals with drug manufacturers.62- 65 If a mean 46% reduction in drug prices occurred, the cost of treating 50% of patients with HCV genotype 1 during the next 5 years could be as high as $29 billion, partly offset by $3 billion in savings in the management of chronic HCV and advanced liver disease."
-----------------------
Cost-effectiveness of Early Treatment of Hepatitis C Virus Genotype 1 by Stage of Liver Fibrosis in a US Treatment-Naive Population
JAMA Intern Med. Published online November 23, 2015
Harinder S. Chahal, PharmD, MSc1,2; Elliot A. Marseille, PhD2,3; Jeffrey A. Tice, MD4; Steve D. Pearson, MD, MSc5; Daniel A. Ollendorf, PhD5; Rena K. Fox, MD4; James G. Kahn, MD, MPH2,6
ABSTRACT
Importance Novel treatments for hepatitis C virus (HCV) infection are highly efficacious but costly. Thus, many insurers cover therapy only in advanced fibrosis stages. The added health benefits and costs of early treatment are unknown.
Objective To assess the cost-effectiveness of (1) treating all patients with HCV vs only those with advanced fibrosis and (2) treating each stage of fibrosis.
Design, Setting, and Participants This study used a decision-analytic model for the treatment of HCV genotype 1. The model used a lifetime horizon and societal perspective and was representative of all US patients with HCV genotype 1 who had not received previous treatment. Comparisons in the model included antiviral treatment of all fibrosis stages (METAVIR [Meta-analysis of Histological Data in Virial Hepatitis] stages F0 [no fibrosis] to F4 [cirrhosis]) vs treatment of stages F3 (numerous septa without cirrhosis) and F4 only and by specific fibrosis stage. Data were collected from March 1 to September 1, 2014, and analyzed from September 1, 2014, to June 30, 2015.
Interventions Six HCV therapy options (particularly combined sofosbuvir and ledipasvir therapy) or no treatment.
Main Outcomes and Measures Cost and health outcomes were measured using total medical costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs), calculated as the difference in costs between strategies divided by the difference in QALYs.
Results We simulated 1000 individuals, but present the results normalized to a single HCV-infected person. In the base-case analysis, among patients receiving 8 or 12 weeks of sofosbuvir-ledipasvir treatment, treating all fibrosis stages compared with treating stages F3 and F4 adds 0.73 QALYs and $28 899, for an ICER of $39 475 per QALY gained.
Treating at stage F2 (portal fibrosis with rare septa) costs $19 833 per QALY gained vs waiting until stage F3;
treating at stage F1 (portal fibrosis without septa), $81 165 per QALY gained compared with waiting until stage F2;
and treating at stage F0, $187 065 per QALY gained compared with waiting until stage F1.
Results for other regimens show a similar pattern.
At base-case drug prices, treating 50% of all eligible US patients with HCV genotype 1 would cost $53 billion.
In sensitivity analyses, the ICER for treating all stages vs treating stages F3 and F4 was most sensitive to cohort age, drug costs, utility values in stages F1 and F2, and percentage of patients eligible for 8-week therapy. Except for patients aged 70 years, the ICER remains less than $100 000 per QALY gained.
A 46% reduction in cost of sofosbuvir-ledipasvir therapy decreases the ICER for treating at all fibrosis stages by 48%.
Conclusions and Relevance In this simulated model, treating HCV infection at early stages of fibrosis appeared to improve health outcomes and to be cost-effective but incurred substantial aggregate costs. The findings may have implications for health care coverage policies and clinical decision making.
INTRODUCTION
In the United States, prevalence of chronic hepatitis C virus (HCV) infection is estimated to be 3.2 million and is the leading cause of liver-related deaths, hepatocellular carcinoma, and liver transplant.1 The primary mode of acquisition is percutaneous exposure to blood, including sharing of injection paraphernalia and a historically contaminated blood supply, which led to a maximum prevalence in the cohort of individuals born from 1945 to 1965.2,3
Previously, treatment of HCV genotype 1 required as long as 48 weeks, with cure rates of 40% to 70% in patients with HCV monoinfection.1 With the introduction of HCV nucleotide analogue nonstructural protein 5A and B inhibitors, such as ledipasvir, ombitasvir, dasabuvir, and sofosbuvir, treatment duration has decreased for most patients to 12 weeks or less, with reduced toxic effects by the exclusion of interferon and often with the exclusion of ribavirin.4 The cure rate with the new therapies generally exceeds 90% and reaches 100% in some subgroups in clinical trials.4- 7 The new drugs cost $1000 per day or more based on the wholesale acquisition price.8 Such costs are prohibitive for many patients and health care systems. Health care professionals may therefore resort to less effective drugs or wait for disease progression before initiating treatment.
Recent cost-effectiveness studies show that treatment with new therapies compared with older drugs is cost-effective for patients with HCV genotype 1, with a net cost per quality-adjusted life-year (QALY) ranging from $10 000 to $30 000.9,10 These studies, however, do not analyze the implications of treatment at various stages of liver fibrosis. Thus, the optimal timing of treatment is unknown.
Despite clinical practice guidelines recommending the new antiviral drugs, some payers require a higher level of fibrosis before authorizing treatment.11- 15 Untreated chronic HCV infection can progress with increasing fibrosis, reaching cirrhosis in 20% to 30% of patients, and related liver complications, including premature death, in a smaller subset.16- 18 Even with viral elimination, some patients may experience disease progression. Earlier treatment might provide important clinical and cost benefits. The objective of this study was to determine the most cost-effective liver fibrosis stage at which to initiate treatment with direct-acting antiviral agents in US treatment-naive patients with HCV genotype 1 infection and was based on commonly accepted thresholds. We present an analysis of a fixed-dose combination of sofosbuvir and ledipasvir (hereinafter, sofosbuvir-ledipasvir). Other regimens are analyzed in eTable 1 in the Supplement.
METHODS
Model Overview
We constructed a decision-analytic model of HCV to examine the clinical outcomes and costs of treatment initiated at different disease stages. The disease states reflect progression through the 5 METAVIR (Meta-analysis of Histological Data in Viral Hepatitis) liver fibrosis stages (F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; and F4, cirrhosis; eTable 1 in the Supplement) to advanced liver disease and regression of liver damage after successful treatment. We simulated a prevalent closed cohort (normalized to a single HCV-infected person) of US treatment-naive patients with HCV genotype 1 until death, tracking costs and QALYs discounted to the present. We validated the model by comparing predictions with the results of empirical natural history studies and prior models. The cost-effectiveness of initiating treatment earlier was calculated by running the model twice, with different start times for antiviral therapy. Additional details are provided below and in the eMethods and eTable 2 in the Supplement, and input variables are available in eTables 3 through 12 in the Supplement.
Data were collected from March 1 to September 1, 2014, and analyzed from September 1, 2014, to June 30, 2015. This project does not meet the criteria for human research and was not required to undergo evaluation by the University of California, San Francisco, Committee on Human Research.
Treatment Characteristics
The model considered therapies for HCV genotype 1 infection by regimens and doses approved by the US Food and Drug Administration.19 The goal of treatment is an undetectable serum level of HCV RNA 12 weeks after completion of therapy, also termed a sustained virologic response (SVR).20 The likelihood of an SVR and treatment discontinuation were determined by meta-analyses of phases 2 and 3 clinical trials stratified by the presence or absence of cirrhosis.15 Discontinuation of therapy was calculated as patient withdrawal from clinical trials for any reason, with an intent-to-treat approach. The discontinuation and SVR rates were subjected to sensitivity analyses.
We present results for sofosbuvir-ledipasvir treatment for 8 or 12 weeks. Duration of sofosbuvir-ledipasvir treatment can be 8 weeks if the baseline viral load is less than 6 million IU/mL with no cirrhosis. Thus 67% of the patients with fibrosis stages F0 to F3 received 8 weeks of treatment and 33% received 12 weeks; all patients with stage F4 received 12 weeks of treatment.15 These proportions were varied in sensitivity analyses. We modeled 6 other HCV treatment regimens (eTable 1 in the Supplement).
Natural History of Chronic HCV
Chronic HCV progression through increasingly severe liver fibrosis is classified with fibrosis scores F0 to F4 (eTable 1 and eFigure 1 in the Supplement). We used these scores and major liver complications to define Markov model disease states (eFigures 1-3 in the Supplement). Transition probabilities between states are based on our review of the published literature. We validated this natural history model by demonstrating correspondence with empirical data on cirrhosis incidence and prior modeling (eTable 2 and eFigure 4 in the Supplement).21,22 The model starts with a prevalent cohort in which the patients are distributed across the 5 stages of fibrosis according to proportions observed in the US population with HCV infection.23
Treatment Strategies
First, we compared treating all patients with treating patients who have disease progression to stage F3 or stage F4, the historical standard at which to consider treatment.11- 15 Second, we compared treatment by fibrosis stage to assess finer distinctions. We considered 6 timing options. The first option treats all patients with HCV. The second option omits treatment of patients at stage F0 but includes those with stages F1 to F4. In this option, the patients with stage F0 disease must progress to stage F1 to be treated. Each successive option (third through fifth) adds 1 fibrosis stage. In the sixth option, for comparison, no treatment is provided.
Progression and Regression After SVR
Achieving SVR slows progression and liver complications by more than 90%.24,25 In addition, some patients experience regression of liver fibrosis after therapy.25- 30 Our model portrays slowed progression via lowered transition probabilities and regression via new transition paths and values.
Patient Population
We simulated 1000 individuals, but present the results normalized to a single HCV-infected person. In our base-case scenario, we portray a cohort of 60-year-old patients (birth year, 1955) weighing 75 kg who are already aware of their HCV infection. The characteristics of patients in the analytic cohort were specified based on data from the 2010 National Health and Nutrition Examination Survey, indicating that 70% of HCV-infected persons were born from 1945 to 1965.31 As this cohort ages, the incidence of complicated liver conditions will increase.32- 34 Other age cohorts ranging from 20 to 70 years are used for scenario analyses. The model does not distinguish patients on the basis of viral concentration, sex, or race, although these factors may affect treatment outcomes.35
Mortality
Mortality for patients with stages F3 and F4 and no SVR is 2.37 times the age-specific background rates from the 2009 US life tables and based on evidence from a prospective cohort study.36,37 Individuals with decompensated cirrhosis and hepatocellular carcinoma have high rates of mortality.25,38 Patients who receive a liver transplant can die of transplant-related complications..25,38 Patients with stages F3 and F4 who achieve SVR have mortality 1.4 times the background population rate based on a meta-analysis of 8 HCV follow-up studies and input from HCV experts.39 Mortality for patients with stages F0 to F2 was assumed to be equal to the rate for the background population.
Costs and Use of Health Care Resources
We adopted a societal perspective, including all direct medical costs for HCV management and therapy. Our intent is to portray societal costs, as approximated by the cost of care sources on which we rely. For unit costs based on reimbursement, the omission of small patient contributions slightly underestimates total costs (a synopsis of each study is provided in the eMethods in the Supplement). Owing to the imprecision of unit cost inputs and the greater uncertainty introduced by estimated rates of patients under current care and use of health care resources, we examined wide ranges of costs in our sensitivity analyses. Costs are in US dollars adjusted to 2014 using the medical component of the US Consumer Price Index.40
Costs of drugs were determined using the wholesale acquisition price from Red Book Online8 in February 2015 and varied widely in sensitivity analyses. In a scenario analysis, drug costs were reduced by 46%, reflecting recent price reductions announced by Gilead Sciences, Inc.41
Annual health care costs associated with a diagnosis of chronic HCV were determined by adapting published empirical data to our cohort of individuals with known chronic HCV.42- 44 Pre-SVR costs ($810 for stages F0-F2, $2150 for stage F3, and $2575 for stage F4) were based on costs from a managed care database that were adjusted for the proportion of known chronic HCV cases estimated to receive health care.44,45 Post-SVR costs for stages F0 to F4 were estimated at 50% lower by taking the midpoint of 2 pre-SVR vs post-SVR cost ratios derived from medical care payment databases in the United States and the United Kingdom.42,46 The model accounted for costs of HCV genotyping, fibrosis staging, and therapy monitoring, including clinic visits, blood and hepatic tests, and HCV RNA quantification. These costs were determined using the Medicare reimbursement schedule and published literature.47- 49 The frequency of monitoring visits and tests was based on HCV treatment guidelines and clinical judgment.11,50
The costs of management of adverse effects were estimated using the frequency of common and serious adverse effects (determined using regimen-specific meta-analysis of clinical trials). We applied the published costs of similar adverse events.15,51
Health State Utility Values
The model incorporates health state utility values by fibrosis stage with and without SVR and transient loss of utility during treatment. Utility values determined from a literature review indicate a utility score of 0.76 in stage F4, 0.79 in stage F3, 0.92 in stage F2, and 0.98 in stages F1 and F0. The SVR raises utility scores to 0.83 in stage F4, 0.86 in stage F3, 0.93 in stage F2, and 1.00 in stages F1 and F0. The utility penalty of treatment was modeled using utility weights of common and serious adverse events, weighted by the frequency of similar events observed in clinical trials.11,50
Model Outcomes
The model produces discounted lifetime QALYs and direct medical costs for each strategy. It then calculates incremental cost-effectiveness ratios (ICERs) as the ratio of the difference in costs between treatment strategies divided by the difference in QALYs. A policy producing an ICER of $150 000 per QALY or less was considered cost-effective; a policy producing an ICER of $50 000 per QALY was considered highly cost-effective. The model was constructed using TreeAge Pro 2014,52 and Excel software53 was used to analyze the data.
Sensitivity Analysis
We conducted 1-way sensitivity analysis on each variable to determine effects on the ICER and 2-way sensitivity analysis on selected variables. The aggregate uncertainty from multiple inputs was quantified via probabilistic sensitivity analysis using uniform distributions. The range in input values was determined by 95% CIs from primary literature sources or meta-analyses. When such data were unavailable, we varied the base-case value from 50% to 150%.
RESULTS
Base Case Results
We present results only for 8 and 12 weeks of sofosbuvir-ledipasvir treatment. Results for other regimens are similar and are presented in eTable 13 in the Supplement.
Treatment of All Stages vs Stages F3 and F4
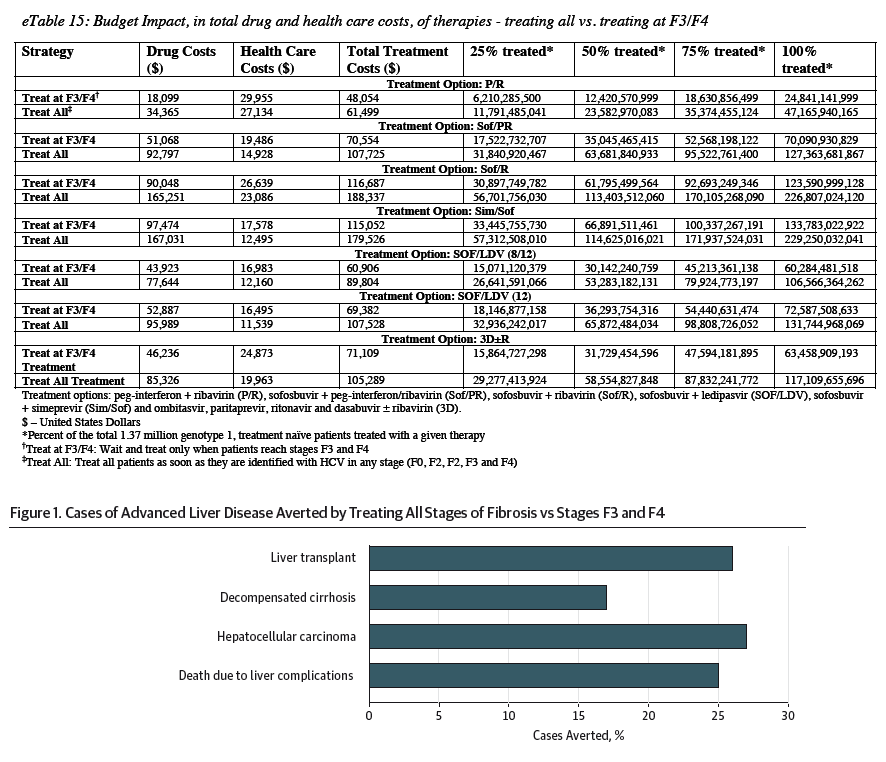
For sofosbuvir-ledipasvir treatment for 8 and 12 weeks, treating all stages of fibrosis compared with treating stages F3 and F4 produced a QALY gain of 0.73 (Table) owing to a higher health state utility value after SVR in early fibrosis (69% of the QALY benefit) and to averted liver complications and death (Figure 1 and eTable 14 in the Supplement; 31% of the QALY benefit). Treating all stages of fibrosis compared with treating stages F3 and F4 increases drug costs by $33 721. An SVR lowers lifetime health care costs by about $5000, resulting in net increased costs of $28 899 for sofosbuvir-ledipasvir treatment (Table and eTable 15 in the Supplement). Treating all stages of fibrosis with sofosbuvir-ledipasvir compared with treating stages F3 and F4 only has net costs per QALY gained of $39 475 (Table).
Treatment by Fibrosis Stage
Sofosbuvir-ledipasvir treatment at earlier stages of fibrosis results in a gain in QALYs (Table). Treating stage F3 increases QALYs by 2.27 compared with treating stage F4; treating stage F2 compared with stage F3 has a QALY gain of 0.55; treating stage F1 compared with stage F2 has a QALY gain of 0.14; and treating stage F0 compared with stage F1 has a QALY gain of 0.03. These QALY gains reflect higher health state utility values in early fibrosis and prevention of advanced liver complications, including premature death.
Treating stage F3 has a $14 798 higher cost compared with waiting until stage F4 (Table). Incremental costs decrease with earlier fibrosis stage comparisons. The cost for treating stage F2 compared with stage F3 is $11 007; for stage F1 compared with stage F2, $11 682; and for stage F0 compared with stage F1, $6210. The higher net costs for initiating treatment in earlier fibrosis stages are driven by the cost of drugs for individuals treated only under broader treatment criteria, which are partially offset by averted costs of care for chronic HCV and advanced liver disease. As shown in the Table, the net cost per QALY gained by sofosbuvir-ledipasvir treatment is $11 252 for treating stage F4 compared with no treatment, $6522 for treating stage F3 compared with stage F4, $19 833 for treating stage F2 compared with stage F3, $81 165 for treating stage F1 compared with stage F2, and $187 065 for treating stage F0 compared with stage F1.
Deterministic Sensitivity Analyses
The inputs to which the ICER for treating all fibrosis stages compared with treating stages F3 and F4 are most sensitive are cohort age and drug cost, with much lower sensitivity to discount rate, utility values in stages F1 and F2 (without SVR), and proportion of patients with disease regression to healthier fibrosis stages (Figure 2). For age, use of a cohort age of 50 years (vs 60 in the base-case analysis) produced the more favorable ICER or $25 443 per QALY gained. At 20 years of age, the ICER is $999 per QALY gained owing to a high likelihood of progression without SVR. At 70 years of age, the ICER is $118 889 per QALY gained owing to the reduced likelihood of untreated chronic HCV causing death. For age analyses, age-dependent fibrosis progression probabilities were used with base-case fibrosis prevalence (eTable 4 in the Supplement). For drug prices, we referenced a recent announcement by Gilead Sciences, Inc (the manufacturer of sofosbuvir-ledipasvir), of a mean price discount to 46%.41 This reduction lowers the cost per QALY gained to $18 807 for treating all stages vs treating stages F3 and F4; similar trends are seen for analysis by fibrosis stage (eTable 16 in the Supplement). Sensitivity analyses on other regimens are available in eTables 17 to 19 and eFigures 5 to 8 in the Supplement.
Probabilistic Sensitivity Analysis
Monte Carlo simulations are shown in Figure 3 as the likelihood of a timing option to be considered cost-effective at different willingness-to-pay thresholds. At a willingness-to-pay threshold of $50 000 per QALY, treating all stages is cost-effective in 74% of simulations. This proportion rises to 96% at a willingness-to-pay threshold of $150 000 per QALY. Probabilistic sensitivity analyses for other regimens are available in eTable 13 and eFigures 9 to 12 in the Supplement.
Budget Impact Analysis
A prior analysis determined 1.32 million treatment-naive persons in the United States would be aware of their HCV infection status by 2014, with an additional 510 000 identified by 2019, for a total of 1.83 million patients.9,54 Assuming that 75% of these patients have HCV genotype 1 (1.37 million), we determined the total cost of drugs required to treat 25%, 50%, 75%, and 100% of these patients during the next 5 years in our budget impact analysis (full details are available in eTable 15 in the Supplement). Figure 4 shows the drug costs of sofosbuvir-ledipasvir treatment. If 50% of eligible patients with HCV genotype 1 (ie, 686 000 patients) are treated regardless of fibrosis stage during the next 5 years, the estimated unadjusted treatment costs are $53 billion. With the 46% reduction in drug prices discussed above, the cost decreases to $29 billion. Alternatively, if 50% of these patients are treated at stages F3 and F4 during the next 5 years, the costs are $30 billion with sofosbuvir-ledipasvir treatment and $16 billion with a 46% decrease in drug prices. Figure 4 depicts the savings in lifetime health care costs exclusive of drug costs gained by treating all stages compared with treating stages F3 and F4, which is $3.3 billion with sofosbuvir-ledipasvir treatment.
DISCUSSION
The new HCV interferon-free therapies offer potentially huge individual and societal benefits but at a large cost. Health plans and health systems concerned about costs frequently require evidence of advanced liver fibrosis before authorizing the new therapies.11- 14 We herein examined the health impact, cost, and cost-effectiveness of earlier treatment.
Although early treatment with sofosbuvir-ledipasvir is expensive, the net cost is substantially lower owing to savings in medical care and the likelihood of later treatment with a delayed treatment policy. Furthermore, we found substantial short- and long-term health gains. Thus, for sofosbuvir-ledipasvir treatment, treating patients at all fibrosis stages compared with waiting for advanced fibrosis is cost-effective (<$50 000 per QALY gained). A detailed analysis of timing of therapy by fibrosis stage shows that treating the disease at as early as stage F1 is cost-effective (ICERs of $50 000-$150 000 per QALY gained) and less than $50 000 per QALY gained when treatment is initiated at stage F2 vs stage F3. The ICER is lower when treatment is initiated at stage F3 compared with waiting for cirrhosis (stage F4). Results are similar for treatment with other new antiviral regimens.
Although the new therapies promise a high SVR, their long-term effects on clinical outcomes are not yet known. Sustained virologic response, a surrogate marker, may not lead to better long-term health outcomes with new treatments. Past studies with older regimens, however, have shown that achieving SVR can result in positive, long-term clinical benefits for patients.55- 60 A 2011 systematic review61 found that achieving SVR can reduce liver-related mortality, incidence of hepatocellular carcinoma, and decompensation and foster regression of fibrosis and cirrhosis.
For budgetary considerations, if only 50% of eligible patients with HCV genotype 1 were to be treated with sofosbuvir-ledipasvir during the next 5 years, the cost of drugs in the United States would be $53 billion at current prices. Many payers negotiate prices, as has been seen with exclusivity deals with drug manufacturers.62- 65 If a mean 46% reduction in drug prices occurred, the cost of treating 50% of patients with HCV genotype 1 during the next 5 years could be as high as $29 billion, partly offset by $3 billion in savings in the management of chronic HCV and advanced liver disease.
Our model has several assumptions and limitations. First, we assumed that patients who achieve SVR have no risk for reinfection with HCV, thus tending to overestimate cost-effectiveness. Second, the model does not consider benefits for patients who receive therapy but do not achieve SVR. Third, the model does not consider the reduction in HCV transmission to seronegative individuals as a consequence of successful therapy. These latter 2 assumptions would underestimate the societal and economic benefits of treatment.
Fourth, the model did not consider extended treatment for patients with slow responses or the repeated treatment of patients who do not achieve SVR. Additional therapy would add to the costs of treatment and possibly improve efficacy. Fifth, the model uses aggregated annualized transition probabilities to simulate progression from one clinical state to the next, adjusted for age but not for other individual traits. This approach focuses the overall simulation on population-level natural history. Individual heterogeneity in chronic hepatitis C virus progression is represented by varying progression rates in sensitivity analyses. Sixth, the analysis took into account only direct medical costs, omitting potential gains in productivity. Seventh, the model considered only patients monoinfected with HCV, excluding coinfections with hepatitis B virus and human immunodeficiency virus.
Eighth, we used meta-analyses of clinical trials to determine SVR and discontinuation rates. The point estimates may differ from those of published phases 2 and 3 trials. We used an intent-to-treat analysis to determine discontinuation rates, and our values may therefore be higher than other estimates. Point estimates from clinical trials may not represent real-world results, which can be lower for SVR and higher for discontinuation rates.66,67 However, our meta-analysis 95% CIs are wide, which allowed us to test SVRs across a wide range in sensitivity analyses.
Ninth, we had imperfect cost data. Costs for HCV care, treatment, and adverse effects are sparsely reported. We attempted to portray societal costs, as measured by the source studies. Our priority in selecting cost sources was addressing a scope of care closely aligned with our model categories. Owing to limited data, we never had to choose between multiple sources for the same data point; therefore, we had to rely on the relevant source's costing methods (eMethods in the Supplement). However, the most challenging aspect of costing was extrapolating from very good costing data for the identified population receiving care (defined by ≥2 HCV codes in a year, thus likely omitting clinically healthy individuals with HCV) to the broader population receiving care and the even larger infected population. The future cost of new HCV therapy is also a major unknown. Because of these various uncertainties, we carefully explored the implications of different cost estimates for the cost-effectiveness outcomes via sensitivity analyses of the cost inputs and of the discount rate that affects the evaluation of future expected costs.
Finally, this model did not simulate changing drug costs over time and how that would affect the cost-effectiveness of early treatment. Market or political forces may result in significantly decreased drug costs in the next several years, and a subset of patients, given the slow progression of HCV, may be treated at a lower cost without a risk for serious clinical progression. These possibilities would make early treatment less cost-effective. However, as in the case of therapies for multiple sclerosis and insulin, the cost of drugs may increase despite being on the market for a number of years and despite new entrants.68,69
CONCLUSIONS
This analysis suggests that treatment with new HCV drugs is cost-effective when started with any evidence of fibrosis (stage F1). Because of the investment required for these drugs, budgetary constraints on health systems typically restrict access to insured patients until they experience higher levels of liver damage or failure of older treatments, and uninsured patients would be unable to receive treatment without patient assistance programs. A reduction in the price will improve cost-effectiveness and increase affordability and access.
|
|
| |
| |
|
|
|