 |
 |
 |
| |
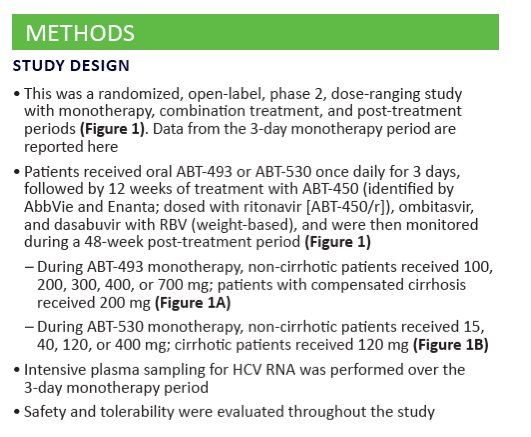
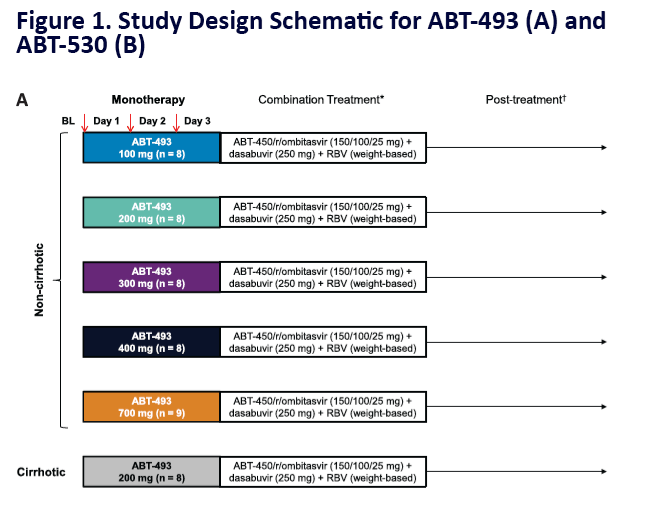
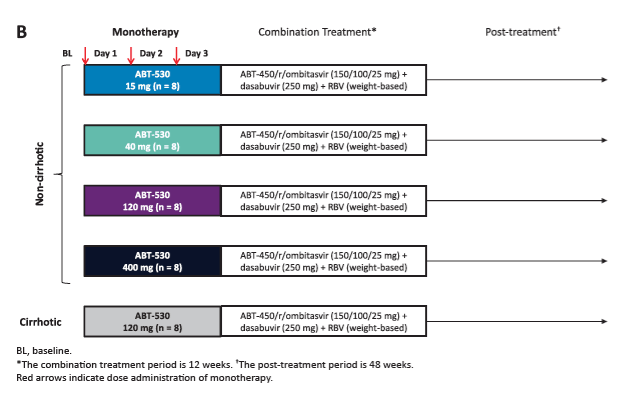
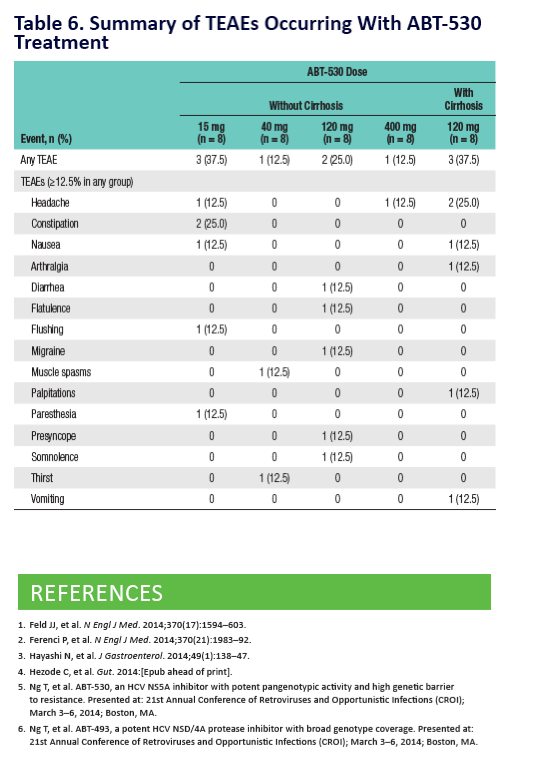
Potent Antiviral Activity of ABT-493 and ABT-530 With 3-Day Monotherapy in Patients With and Without Compensated Cirrhosis With Hepatitis C Virus (HCV) Genotype 1 Infection
|
| |
| |
Reported by Jules Levin
AASLD November 7-11 2014 Boston, MA
Eric J Lawitz1, William D O'Riordan2, Bradley L Freilich3, Terry D Box4, J Scott Overcash2, Wei Liu5, Andrew Campbell5, Chih-Wei Lin5, Sandra Lovell5, Bett y Yao5, Armen Asatryan5, Jens Kort5
1Texas Liver Insti tute, University of Texas Health Science Center, San Antonio, Texas, United States; 2eStudySite, San Diego, California, United States; 3Kansas City Gastroenterology & Hepatology, Kansas City, Missouri, United States; 4Clinical Research Centers of America, Murray, Utah, United States; 5AbbVie Inc., North Chicago, Illinois, United States
This may have flown under your radar, Abbvie's EASL press release said
http://www.natap.org/2015/EASL/EASL_01.htm:
AbbVie's ongoing HCV pipeline development program focuses on investigating a pan-genotypic, ribavirin (RBV)-free, once-daily treatment that may also allow for treatment durations of as little as eight weeks. Preliminary results from a Phase 2b study (n=79) of ABT-493 and ABT-530 in non-cirrhotic GT1 patients receiving the RBV-free recommended regimen for 12 weeks demonstrated a sustained virologic response rate at four weeks post treatment (SVR4) of 99 percent (n=78/79). These results, announced for the first time today, included both GT1a and GT1b, treatment-naļve and pegylated-interferon and RBV prior null responders. Patients across both study arms were randomized to receive ABT-493 (200mg) and either 120mg or 40mg of ABT-530. To date, the most common (>5 percent) adverse reactions were fatigue, headache, nausea, diarrhea and anxiety. Data from these Phase 2b studies of ABT-493 and ABT-530 will not be presented at ILC 2015, and will be released at future medical congresses.
ABT-493, a Potent HCV NS3/4A Protease Inhibitor With Broad Genotype Coverage......
http://www.natap.org/2014/CROI/croi_14.htm
A Next Generation HCV DAA Combination: Potent, Pangenotypic Inhibitors ABT-493 and ABT-530 With High Barriers to Resistance......
http://www.natap.org/2014/AASLD/AASLD_51.htm
ABT-530, an HCV NS5A Inhibitor With Potent Pangenotypic Activity and High Genetic Barrier to Resistance.....
http://www.natap.org/2014/CROI/croi_11.htm


|
| |
|
 |
 |
|
|