| |
HIV status and the risk of ischemic stroke among men
|
| |
| |
Download the PDF here
......"HIV infection is associated with an increased ischemic stroke risk among HIV-infected compared with demographically and behaviorally similar uninfected male veterans.......Compared with uninfected veterans, HIV-infected veterans with a CD4+ T-cell count <200 cells/mm3 (HR = 1.66, 95% CI 1.30-2.12) or HIV-1 RNA ≥500 copies/mL (HR = 1.36, 95% CI 1.15-1.63) were at highest risk of incident ischemic stroke......Mean (SD) age at stroke event was 57.2 (9.8) and 58.4 (9.3) years"
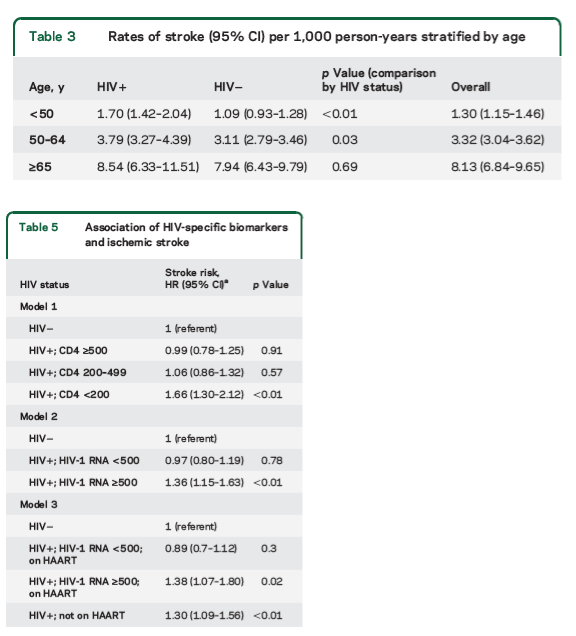
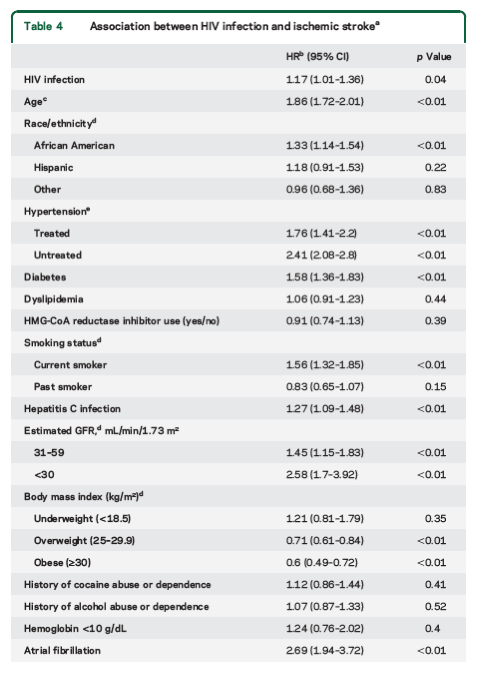
Rates of stroke increased with age (table 3). CD4 & detectable viral load are associated with ischemic stroke (table 5). Table 4 shows associations between & HIV & ischemic stroke: HIV, age, African-American, untreated hypertension, treated hypertension, diabetes, current smoker, HCV, (renal/kidney function) eGFR <59, overweight, obese, atrial fibrillation.
Neurology May 12, 2015
For the Veterans Aging Cohort Study
"Compared with uninfected veterans with similar (or higher) comorbidity burden, veterans with HIV infection had a significantly increased risk of ischemic stroke. These associations persisted after adjusting for traditional ischemic stroke risk factors, comorbid diseases, and substance abuse or dependence. Age, African American race/ethnicity, diabetes, hypertension, atrial fibrillation, smoking, alcohol abuse or dependence, renal disease, and CD4+ T-cell count <200 cells/mm3 were associated with ischemic stroke among veterans with HIV infection. ......After adjusting for demographics, ischemic stroke risk factors, comorbid diseases, and substance abuse or dependence, HIV-infected veterans had a significantly increased risk of ischemic stroke compared with uninfected veterans (HR = 1.17 [1.01-1.36]; p = 0.04).......Compared with uninfected veterans, HIV-infected veterans with a CD4+ T-cell count <200 cells/mm3 (HR = 1.66, 95% CI 1.30-2.12) or HIV-1 RNA ≥500 copies/mL (HR = 1.36, 95% CI 1.15-1.63) were at highest risk of incident ischemic stroke (table 5). HIV-infected veterans with unsuppressed viremia taking HAART or any HIV-infected veteran not taking HAART at baseline had higher risk of stroke compared with uninfected veterans (table 5).
Stroke rates per 1,000 person-years increased with age (table 3) and were higher for veterans with HIV infection (2.79, 95% CI 2.51-3.10) than for veterans without infection (2.24, 95% CI 2.06-2.43) (incidence rate ratio 1.25 [1.09-1.43]; p < 0.01). After adjusting for demographics, ischemic stroke risk factors, comorbid diseases, and substance abuse or dependence, HIV-infected veterans had a significantly increased risk of ischemic stroke compared with uninfected veterans (HR = 1.17 [1.01-1.36]; p = 0.04) (table 4). Other risk factors significantly associated with ischemic stroke were age, African American race/ethnicity, diabetes, hypertension, atrial fibrillation, HCV infection, current smoking, overweight and obesity, and renal disease (table 4).
Our data suggest that while traditional risk factors are important for stroke pathogenesis (especially hypertension), HIV infection, especially when less well controlled (i.e., lower CD4 counts, higher HIV-1 RNA), contributes to stroke risk independently of but in addition to traditional risk factors, substance abuse or dependence, and sociodemographic characteristics. These results have important implications in the management of those aging with HIV, and may help guide intervention strategies to decrease ischemic stroke risk in this higher-risk population.
With the advent of HAART and ever-increasing life expectancy, people with HIV infection are living long enough to develop traditional stroke risk factors, the ill effects of polysubstance use, and HAART-associated toxicities.
Consequently, increasing age, nonwhite race, hypertension, diabetes, atrial fibrillation, current smoking, a history of alcohol abuse or dependence, and renal disease were significantly associated with increased risk of ischemic stroke in our analysis. The observation that most of these risk factors for ischemic stroke were less prevalent among veterans with HIV infection at baseline may indicate that there are additional HIV-specific mechanisms of increased stroke risk independent of these risk factors.
After excluding participants with known baseline CVD (n = 20,502) and women (n = 2,187), our final sample was 76,835 participants (33% HIV-infected). Compared with uninfected veterans, those with HIV infection had a higher prevalence of dyslipidemia, current smoking, cocaine abuse and dependence, renal disease, and anemia, and a lower prevalence of hypertension, diabetes, obesity, atrial fibrillation, and HMG-CoA reductase inhibitor use (table 1). Alcohol abuse and dependence was similar between both groups (table 1). Among the veterans with HIV infection, more than half had HIV-1 RNA ≥500 copies/mL and almost 30% had CD4+ T-cell counts <200 cells/mm3 at baseline (table 1).
During a median follow-up of 5.9 (interquartile range 3.5-6.6) years, there were 910 incident ischemic stroke events, with 340 events occurring among veterans with HIV infection (table 2). Among those with ischemic stroke, there were no differences in race/ethnicity, smoking status, atrial fibrillation, or alcohol abuse and dependence or HMG-CoA reductase inhibitor use by HIV status. Mean (SD) age at stroke event was 57.2 (9.8) and 58.4 (9.3) years and mean (SD) time to stroke event was 3.4 (1.8) and 3.5 (1.7) years for HIV-infected and uninfected participants, respectively (p > 0.05 for both comparisons). Compared with uninfected veterans, HIV-infected veterans had a lower prevalence of hypertension, diabetes, and obesity, and a higher prevalence of dyslipidemia, HCV infection, cocaine abuse and dependence, renal disease, and anemia. Among the veterans with HIV infection, 60% had HIV-1 RNA ≥500 copies/mL and 36% had CD4+ T-cell counts <200 cells/mm3 at baseline (table 2).
Stroke rates per 1,000 person-years increased with age (table 3) and were higher for veterans with HIV infection (2.79, 95% CI 2.51-3.10) than for veterans without infection (2.24, 95% CI 2.06-2.43) (incidence rate ratio 1.25 [1.09-1.43]; p < 0.01). After adjusting for demographics, ischemic stroke risk factors, comorbid diseases, and substance abuse or dependence, HIV-infected veterans had a significantly increased risk of ischemic stroke compared with uninfected veterans (HR = 1.17 [1.01-1.36]; p = 0.04) (table 4). Other risk factors significantly associated with ischemic stroke were age, African American race/ethnicity, diabetes, hypertension, atrial fibrillation, HCV infection, current smoking, overweight and obesity, and renal disease (table 4).
Compared with uninfected veterans, HIV-infected veterans with a CD4+ T-cell count <200 cells/mm3 (HR = 1.66, 95% CI 1.30-2.12) or HIV-1 RNA ≥500 copies/mL (HR = 1.36, 95% CI 1.15-1.63) were at highest risk of incident ischemic stroke (table 5). HIV-infected veterans with unsuppressed viremia taking HAART or any HIV-infected veteran not taking HAART at baseline had higher risk of stroke compared with uninfected veterans (table 5).
Immunosuppression and HIV viral replication have been associated with increased rates of stroke and risk of CVD.3,5,18,32 Similarly, we found that stroke rates and risk were higher among those with lower CD4 counts or higher HIV-1 RNA and similar among those with higher CD4+ T-cell count or lower HIV-1 RNA compared with uninfected veterans. This highlights the need for continued emphasis of early and consistent HIV control when placed in the larger context that viral suppression occurs only in an estimated 28% of those living with HIV in the United States
People infected with HIV are living longer. Aging, conditions associated with aging (e.g., hypertension and atrial fibrillation), and HAART-related toxicities (e.g., dyslipidemia, impaired glucose tolerance) may all increase the risk of ischemic stroke.8,9 Hepatitis C virus (HCV) infection, common among HIV-infected people, may also increase vascular risk.1,10,-,12 Duration of HIV infection and its associated chronic inflammatory state may also increase the risk of stroke.13"
--------------------------
HIV status and the risk of ischemic stroke among men
Neurology May 12, 2015
Jason J. Sico, MD Chung-Chou H. Chang, PhD Kaku So-Armah Amy C. Justice, MD, PhD Elaine Hylek, MD, MPH Melissa Skanderson, MS Kathleen McGinnis, MS Lewis H. Kuller, MD, DrPH Kevin L. Kraemer, MD, MSc David Rimland, MD Matthew Bidwell Goetz, MD Adeel A. Butt, MD, MS Maria C. Rodriguez-Barradas, MD Cynthia Gibert, MD David Leaf, MD, MPH Sheldon T. Brown, MD Jeffrey Samet, MD, MA, MPH Lewis Kazis, ScD Kendall Bryant, PhD Matthew S. Freiberg, MD, MSc
For the Veterans Aging Cohort Study
From the VA Connecticut Health Care System (J.J.S., A.C.J., M.S., K.M.), West Haven Veterans Administration Medical Center, West Haven; Yale University School of Medicine (J.J.S., K.S.-A., A.C.J.), New Haven, CT; University of Pittsburgh School of Medicine (C.-C.H.C., K.L.K., A.A.B.); University of Pittsburgh Graduate School of Public Health (C.-C.H.C., L.H.K.), Pittsburgh, PA; Boston Medical Center (E.H.), MA; Emory University School of Medicine and Atlanta Veterans Administration Medical Center (D.R.), Atlanta, GA; David Geffen School of Medicine at UCLA and the VA Greater Los Angeles Health Care System (M.B.G., D.L.), Los Angeles, CA; VA Pittsburgh Health Care System (A.A.B.), Pittsburgh, PA; Michael E. DeBakey Veterans Administration Medical Center and Baylor College of Medicine (M.C.R.-B.), Houston, TX; Washington DC Veterans Administration Medical Center and George Washington University School of Medicine (C.G.), Washington, DC; James J. Peters VA (S.T.B.), Bronx; Mount Sinai School of Medicine (S.T.B.), New York, NY; Boston University School of Medicine (J.S.), MA; Center for the Assessment of Pharmaceutical Practices (L.K.), Department of Health Policy and Management, Boston University School of Public Health; Center for Healthcare Organization and Implementation Research (L.K.), a Center for Innovation, Veterans Administration Medical Center, Bedford, MA; National Institute on Alcohol Abuse and Alcoholism (K.B.), Bethesda, MD; and Vanderbilt University School of Medicine and the Nashville Veterans Affairs Medical Center (M.S.F.), Nashville, TN.
Abstract
Objective: Given conflicting data regarding the association of HIV infection and ischemic stroke risk, we sought to determine whether HIV infection conferred an increased ischemic stroke risk among male veterans.
Methods: The Veterans Aging Cohort Study-Virtual Cohort consists of HIV-infected and uninfected veterans in care matched (1:2) for age, sex, race/ethnicity, and clinical site. We analyzed data on 76,835 male participants in the Veterans Aging Cohort Study-Virtual Cohort who were free of baseline cardiovascular disease. We assessed demographics, ischemic stroke risk factors, comorbid diseases, substance use, HIV biomarkers, and incidence of ischemic stroke from October 1, 2003, to December 31, 2009.
Results: During a median follow-up period of 5.9 (interquartile range 3.5-6.6) years, there were 910 stroke events (37.4% HIV-infected). Ischemic stroke rates per 1,000 person-years were higher for HIV-infected (2.79, 95% confidence interval 2.51-3.10) than for uninfected veterans (2.24 [2.06-2.43]) (incidence rate ratio 1.25 [1.09-1.43]; p < 0.01). After adjusting for demographics, ischemic stroke risk factors, comorbid diseases, and substance use, the risk of ischemic stroke was higher among male veterans with HIV infection compared with uninfected veterans (hazard ratio 1.17 [1.01-1.36]; p = 0.04).
Conclusions: HIV infection is associated with an increased ischemic stroke risk among HIV-infected compared with demographically and behaviorally similar uninfected male veterans.
-----------------
GLOSSARY
CI=confidence interval;
CVD=cardiovascular disease;
HAART=highly active antiretroviral therapy;
HCV=hepatitis C virus;
HMG-CoA=3-hydroxy-3-methylglutaryl-coenzyme A;
HR=hazard ratio;
ICD-9=International Classification of Diseases, Ninth Revision;
ICD-9-CM=International Classification of Diseases, Ninth Revision, Clinical Modification;
VA=Veterans Affairs;
VACS-VC=Veterans Aging Cohort Study-Virtual Cohort
-------------------
HIV infection in the highly active antiretroviral therapy (HAART) era is associated with coronary heart disease and hemorrhagic stroke.1,-,5 Most investigations regarding HIV and ischemic stroke come from pre-HAART studies and were limited to younger patients and those with AIDS.6,7
People infected with HIV are living longer. Aging, conditions associated with aging (e.g., hypertension and atrial fibrillation), and HAART-related toxicities (e.g., dyslipidemia, impaired glucose tolerance) may all increase the risk of ischemic stroke.8,9 Hepatitis C virus (HCV) infection, common among HIV-infected people, may also increase vascular risk.1,10,-,12 Duration of HIV infection and its associated chronic inflammatory state may also increase the risk of stroke.13 Large studies comparing ischemic stroke risk in HIV-infected patients with demographically and behaviorally similar uninfected patients in the HAART era are lacking.14,-,16 Recently, HIV infection was found to be an independent risk factor for acute ischemic stroke among women, but not men,17 conflicting with findings from another large, recent study with an uninfected comparator group from within the same health care system18 and a cohort of HIV-infected and uninfected men.16 These important studies included an uninfected comparator group of subjects who had a lower burden of comorbid disease associated with stroke despite age, sex, and race matching.
We examined the independent association between HIV and ischemic stroke in a relatively older, male population in the early HAART era, with uninfected comparators who had a similar (or higher) burden of comorbid disease.
METHODS
Patients.
The Veterans Aging Cohort Study-Virtual Cohort (VACS-VC) is a cohort of veterans with HIV infection each matched to 2 uninfected veterans on age, sex, race/ethnicity, and geographical location. Participants were identified from United States Veterans Affairs (VA) administrative data starting in 1998 using a modified existing algorithm.19 This cohort consists of data from the immunology case registry; the VA HIV registry; the pharmacy benefits management database; the decision support system, a national database of VA clinical and laboratory data; and the National Patient Care Database, and health factors data collected from physician clinical reminders within the VA electronic medical record system. The institutional review boards at the University of Pittsburgh, Yale University, and the West Haven VA Medical Center approved this study.
All participants alive and enrolled in the VACS-VC on or after 2003 (n = 99,688) were eligible for the current study. Baseline was a participant's first clinical encounter on or after April 1, 2003. After excluding women and participants with baseline cardiovascular disease (CVD) (i.e., coronary heart disease, acute myocardial infarction, cardiovascular revascularization, or heart failure) and stroke (ischemic/hemorrhagic), our final sample size was 76,835 veterans (33% HIV-infected). Prevalent CVD and stroke were identified by the occurrence of ICD-9-CM20 codes at any time before and up to 6 months after their baseline date. All participants in the final sample were followed from their baseline date to either an ischemic stroke event, death, or the date of last follow-up. All analyses were truncated to December 31, 2009, to correspond with the end of our event data.
Independent variable.
The presence of HIV infection was defined as a participant with ≥1 inpatient and/or ≥2 outpatient ICD-9 codes for HIV infection and confirmed by the participant's presence in the VA immunology case registry.19
Dependent variable.
Incident ischemic stroke was defined as ≥1 inpatient and/or ≥2 outpatient ICD-9 codes for the diagnosis. These included occlusion and stenosis of precerebral arteries (433.x1), occlusion of cerebral arteries, excluding cerebral thrombosis/embolism without infarction (434, excluding 434.x0), and acute but ill-defined cerebrovascular disease (436).21 Prior work in the Cardiovascular Health Study and elsewhere has demonstrated high agreement of these codes with formal chart adjudication (positive predictive value = 81.6%-90.0%, K = 0.86).2,20,22 Incident hemorrhagic strokes defined by ICD-9 codes were excluded, except where an ischemic stroke later underwent hemorrhagic transformation.
Covariates.
Sociodemographic data included age in 10-year increments, sex, and race/ethnicity. Hypertension, diabetes, dyslipidemia, renal disease, and anemia were measured using outpatient and clinical laboratory data closest to the baseline date. Medications including 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor use and antiretroviral therapy were based on pharmacy data, and body mass index and smoking were measured from the health factors data, which contains information collected from clinical reminders that providers are required to complete on patients. Hypertension was defined as nonhypertensive (<140/90 mm Hg and no evidence of medication therapy), controlled hypertensive (<140/90 mm Hg but on medication), and uncontrolled hypertension (>140/90 mm Hg).23 Blood pressure measurement was the average of the 3 routine outpatient clinical measurements closest to the baseline date. Diabetes was defined using a combination of an abnormal glucose measurement, use of insulin or oral hypoglycemic agents, and/or ICD-9 codes.24 Dyslipidemia was defined as triglycerides ≥150 mg/dL, high-density lipoprotein <40 mg/dL, or low-density lipoprotein ≥160 mg/dL. HMG-CoA reductase inhibitor use was within 180 days of the baseline date. Smoking was defined as current, past, and never smoking based on documentation from the VA electronic medical record health factor dataset. Our prior work demonstrates a high agreement between participant self-completed smoking data from the VACS and health factors data (agreement = 79.5%, K = 0.66).25 HCV infection was defined as a positive HCV antibody test or ≥1 inpatient and/or ≥2 outpatient codes for this diagnosis.26 Alcohol abuse or dependence, cocaine abuse or dependence, and atrial fibrillation (427.31) were defined using ICD-9 codes.27,28
We also collected data on baseline CD4+ cell counts, HIV-1 RNA levels, and HAART. Baseline CD4+ cell counts and HIV-1 RNA levels were collected within 180 days of our baseline date. Baseline antiretroviral therapy use was defined as use of nonnucleoside reverse transcriptase inhibitor, nucleoside reverse transcriptase inhibitor, and protease inhibitor, within 180 days of the baseline date. We have previously shown within a nested sample of veterans with HIV infection that 98% receive antiretroviral therapy within the VA.19
Statistical analysis.
We assessed all descriptive statistics using t tests or its nonparametric counterpart for continuous variables and χ2 test for categorical variables. Among those with ischemic stroke, risk factors were compared by HIV status. We calculated age- and race/ethnicity-adjusted incident ischemic stroke rates per 1,000 person-years. Cox proportional hazard models were used to estimate the hazard ratio (HR) and confidence intervals (CIs) for incident ischemic stroke associated with HIV status after adjusting for demographics, traditional ischemic stroke risk factors, comorbidities, and substance use. Secondary analyses stratified HIV status by CD4+ T-cell count, HIV-1 RNA levels, and HAART use. The proportional hazards assumption was assessed using the Grambsch-Therneau method and was not violated for any analyses.29 Given the low percentage of women in the cohort and prior work suggesting an interaction by sex on the effect of HIV on stroke risk, women were excluded from these analyses. Multiple imputation techniques were used to include missing data into these analyses; these techniques generated 5 datasets with complete covariance values, increasing the robustness and efficiency of estimated HRs.
Standard protocol approvals, registrations, and patient consents.
The development of VACS-VC was approved by the institutional review boards of the VA Connecticut Healthcare System and Yale University School of Medicine, granted a waiver of informed consent, and is HIPAA (Health Insurance Portability and Accountability Act)-compliant.
RESULTS
After excluding participants with known baseline CVD (n = 20,502) and women (n = 2,187), our final sample was 76,835 participants (33% HIV-infected). Compared with uninfected veterans, those with HIV infection had a higher prevalence of dyslipidemia, current smoking, cocaine abuse and dependence, renal disease, and anemia, and a lower prevalence of hypertension, diabetes, obesity, atrial fibrillation, and HMG-CoA reductase inhibitor use (table 1). Alcohol abuse and dependence was similar between both groups (table 1). Among the veterans with HIV infection, more than half had HIV-1 RNA ≥500 copies/mL and almost 30% had CD4+ T-cell counts <200 cells/mm3 at baseline (table 1).
During a median follow-up of 5.9 (interquartile range 3.5-6.6) years, there were 910 incident ischemic stroke events, with 340 events occurring among veterans with HIV infection (table 2). Among those with ischemic stroke, there were no differences in race/ethnicity, smoking status, atrial fibrillation, or alcohol abuse and dependence or HMG-CoA reductase inhibitor use by HIV status. Mean (SD) age at stroke event was 57.2 (9.8) and 58.4 (9.3) years and mean (SD) time to stroke event was 3.4 (1.8) and 3.5 (1.7) years for HIV-infected and uninfected participants, respectively (p > 0.05 for both comparisons). Compared with uninfected veterans, HIV-infected veterans had a lower prevalence of hypertension, diabetes, and obesity, and a higher prevalence of dyslipidemia, HCV infection, cocaine abuse and dependence, renal disease, and anemia. Among the veterans with HIV infection, 60% had HIV-1 RNA ≥500 copies/mL and 36% had CD4+ T-cell counts <200 cells/mm3 at baseline (table 2).
Stroke rates per 1,000 person-years increased with age (table 3) and were higher for veterans with HIV infection (2.79, 95% CI 2.51-3.10) than for veterans without infection (2.24, 95% CI 2.06-2.43) (incidence rate ratio 1.25 [1.09-1.43]; p < 0.01). After adjusting for demographics, ischemic stroke risk factors, comorbid diseases, and substance abuse or dependence, HIV-infected veterans had a significantly increased risk of ischemic stroke compared with uninfected veterans (HR = 1.17 [1.01-1.36]; p = 0.04) (table 4). Other risk factors significantly associated with ischemic stroke were age, African American race/ethnicity, diabetes, hypertension, atrial fibrillation, HCV infection, current smoking, overweight and obesity, and renal disease (table 4).
Compared with uninfected veterans, HIV-infected veterans with a CD4+ T-cell count <200 cells/mm3 (HR = 1.66, 95% CI 1.30-2.12) or HIV-1 RNA ≥500 copies/mL (HR = 1.36, 95% CI 1.15-1.63) were at highest risk of incident ischemic stroke (table 5). HIV-infected veterans with unsuppressed viremia taking HAART or any HIV-infected veteran not taking HAART at baseline had higher risk of stroke compared with uninfected veterans (table 5).
DISCUSSION
Compared with uninfected veterans with similar (or higher) comorbidity burden, veterans with HIV infection had a significantly increased risk of ischemic stroke. These associations persisted after adjusting for traditional ischemic stroke risk factors, comorbid diseases, and substance abuse or dependence. Age, African American race/ethnicity, diabetes, hypertension, atrial fibrillation, smoking, alcohol abuse or dependence, renal disease, and CD4+ T-cell count <200 cells/mm3 were associated with ischemic stroke among veterans with HIV infection.
The increased rates and risk of ischemic stroke among veterans with HIV infection are consistent with findings from 2 recent studies with uninfected comparators from the same health care system (Kaiser Permanente and Partners Healthcare, respectively)17,18 and for findings among men in the Multicenter AIDS Cohort Study.16 Absolute stroke rates in our cohort were higher than those reported in 2 former studies possibly because of the higher prevalence of ischemic stroke risk factors among our participants. Contrary to our findings and to those by the Kaiser Permanente group and in the Multicenter AIDS Cohort Study, others did not find a significant association between HIV status and stroke risk among men in sex-stratified analyses. Reasons for the discrepancy may include differences between the cohorts. For example, our cohort participants were older at baseline (49 vs 41 years) and more likely to be African American (47% vs 22%), 2 important nonmodifiable ischemic stroke risk factors (tables 3 and 4). In addition, we excluded veterans with prevalent heart disease while others adjusted for prevalent heart disease increasing the potential for unmeasured confounding because of ascertainment bias.
The present study extends the knowledge gained from these prior studies. First, it provides data on an aging HIV-infected cohort experiencing stroke outcomes. Data on absolute and relative stroke risk from early waves of aging HIV-infected people are sparse and will improve prioritization of stroke-prevention and risk-reduction efforts in this population. Second, this aging cohort is compared with an aging HIV-uninfected cohort with a similar or higher burden of stroke risk factors (e.g., hypertension, diabetes, atrial fibrillation) at baseline. Comparing stroke risk in an HIV-infected population with that from an uninfected population with a significantly lower burden of stroke risk factors may overestimate the association of HIV status and incident stroke.
Few other large epidemiologic studies have addressed incident ischemic stroke risk among HIV-infected and uninfected people in the same cohort. Prior work was done in the pre-HAART era or limited by the low number of events, lack of a demographically and behaviorally similar HIV-uninfected comparator group, or lack of adjustment for ischemic stroke risk factors.4,14,15,30 The results of the present study are largely consistent with those from prior studies.
The mechanisms by which HIV infection confers an increased risk of ischemic stroke in the HAART era are unclear and likely multifactorial. While processes that caused strokes in the pre-HAART era (e.g., opportunistic infections, malignancies) may still affect patients who experience immunodeficiency, they may be less relevant in patients with better HIV control.10,31 With the advent of HAART and ever-increasing life expectancy, people with HIV infection are living long enough to develop traditional stroke risk factors, the ill effects of polysubstance use, and HAART-associated toxicities.9,10,15,31 Consequently, increasing age, nonwhite race, hypertension, diabetes, atrial fibrillation, current smoking, a history of alcohol abuse or dependence, and renal disease were significantly associated with increased risk of ischemic stroke in our analysis. The observation that most of these risk factors for ischemic stroke were less prevalent among veterans with HIV infection at baseline may indicate that there are additional HIV-specific mechanisms of increased stroke risk independent of these risk factors.
Immunosuppression and HIV viral replication have been associated with increased rates of stroke and risk of CVD.3,5,18,32 Similarly, we found that stroke rates and risk were higher among those with lower CD4 counts or higher HIV-1 RNA and similar among those with higher CD4+ T-cell count or lower HIV-1 RNA compared with uninfected veterans. This highlights the need for continued emphasis of early and consistent HIV control when placed in the larger context that viral suppression occurs only in an estimated 28% of those living with HIV in the United States.33
Our current study has limitations that warrant discussion. The use of stroke ICD-9 codes may have resulted in some misclassification. However, prior studies have reported that these codes have high agreement with formally adjudicated outcomes and are sensitive and specific within the veteran stroke population.2,20,21 Our analyses did not adjust for aspirin use. Aspirin use is incompletely captured using VA pharmacy records because it is frequently purchased over the counter. Aspirin use has been associated with decreased risk of stroke among patients with HIV infection.17 In one VA study that used administrative and chart review data to examine the quality of poststroke care, the median standardized rate of prescribing antithrombotic agents among eligible patients was 97% (27th, 75th percentiles: 96%, 98%).34 This suggests that the absolute number of poststroke veterans with differential aspirin use by HIV status is likely to be small assuming such a bias existed. Imaging data were not available to explore the etiology of ischemic stroke, stroke severity, or stroke subtype. This becomes important, especially among patients with lower CD4 counts and higher degrees of viral replication, because they may also be susceptible to ischemic strokes from opportunistic infections and malignancies. We also cannot comment on reasons patients in our cohort did not obtain complete viral suppression; etiologies such as medication resistance and noncompliance should be further explored in this group at high risk of cerebrovascular disease. We did not control for recent use of cocaine. As with any observational study, we cannot exclude the possibility of residual or unmeasured confounding.
Our data suggest that while traditional risk factors are important for stroke pathogenesis (especially hypertension), HIV infection, especially when less well controlled (i.e., lower CD4 counts, higher HIV-1 RNA), contributes to stroke risk independently of but in addition to traditional risk factors, substance abuse or dependence, and sociodemographic characteristics. These results have important implications in the management of those aging with HIV, and may help guide intervention strategies to decrease ischemic stroke risk in this higher-risk population.
|
|
| |
| |
|
|
|