| |
New CDC PrEP Guidelines May 14 2015
|
| |
| |
Download the PDF here
Pre-exposure prophylaxis, or PrEP, is a way for people who do not have HIV to help prevent HIV infection by taking a pill every day. The pill contains two medicines that are also used, in combination with other medicines, to treat HIV. When someone is exposed to HIV through sex or injection drug use, PrEP can help stop the virus from establishing a permanent infection. When used consistently, PrEP has been shown to greatly reduce the risk of HIV infection in people who are at substantial risk. PrEP is much less effective when it is not taken consistently. PrEP is a powerful HIV prevention tool, and can be combined with condoms and other prevention methods to provide even greater protection than when used alone. People who use PrEP must commit to taking the drug daily and seeing their health care provider every 3 months for HIV testing and other follow-up.
Guidelines for PrEP Use
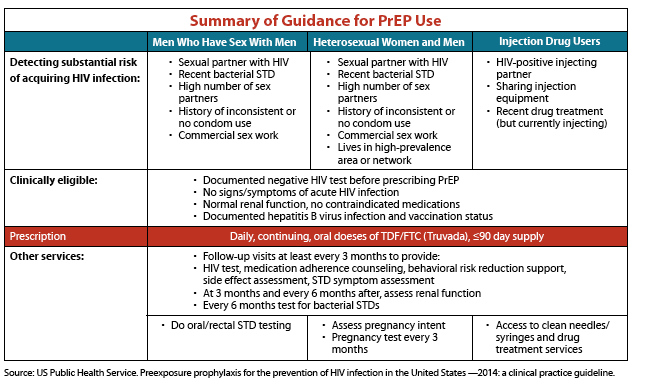
The new federal guidelines for health care providers recommend that PrEP be considered for people who are HIV-negative and at substantial risk for HIV infection.
For sexual transmission, this includes anyone who is in an ongoing relationship with an HIV-positive partner. It also includes anyone who 1) is not in a mutually monogamous relationship with a partner who recently tested HIV-negative, and 2) is a gay or bisexual man who has had anal sex without a condom or been diagnosed with an STD in the past 6 months; or heterosexual man or woman who does not regularly use condoms during sex with partners of unknown HIV status who are at substantial risk of HIV infection (e.g., people who inject drugs or have bisexual male partners).
For people who inject drugs, this includes those who have injected illicit drugs in past 6 months and who have shared injection equipment or been in drug treatment for injection drug use in the past 6 months.
Health care providers should also discuss PrEP with heterosexual couples in which one partner is HIV-positive and the other is HIV-negative as one of several options to protect the partner who is HIV-negative during conception and pregnancy.
For a summary of clinical indications and treatment recommendations for PrEP, see the Table on the next page.
PrEP Medicines
Most PrEP clinical trials have tested a combination of two antiretroviral drugs, tenofovir disoproxil fumarate (also called TDF, or tenofovir) and emtricitabine (also called FTC), taken in a single pill daily for HIV prevention. This combination pill (brand name Truvada) was approved by the US Food and Drug Administration (FDA) for use as an HIV treatment in 2004, and was approved as PrEP in July 2012. Some clinical studies have also evaluated the use of tenofovir on its own as a preventive drug, but this drug
alone is not FDA-approved for PrEP.
--------------------------
Pre-Exposure Prophylaxis (PrEP)
http://www.cdc.gov/hiv/prevention/research/prep/
Pre-exposure prophylaxis, or PrEP, is a way for people who do not have HIV but who are at substantial risk of getting it to prevent HIV infection by taking a pill every day. The pill (brand name Truvada) contains two medicines (tenofovir and emtricitabine) that are used in combination with other medicines to treat HIV. When someone is exposed to HIV through sex or injection drug use, these medicines can work to keep the virus from establishing a permanent infection.
When taken consistently, PrEP has been shown to reduce the risk of HIV infection in people who are at high risk by up to 92%. PrEP is much less effective if it is not taken consistently.
PrEP is a powerful HIV prevention tool and can be combined with condoms and other prevention methods to provide even greater protection than when used alone. But people who use PrEP must commit to taking the drug every day and seeing their health care provider for follow-up every 3 months.
Federal PrEP Guidelines
On May 14, 2014, the US Public Health Service released the first comprehensive clinical practice guidelines for PrEP . The guidelines were developed by federal inter-agency working group led by CDC, and reflect input from providers, HIV patients, partners, and affected communities. The new guidelines
· Provide clear criteria for determining a person’s HIV risk and indications for PrEP use.
· Require that patients receive HIV testing to confirm negative status before starting PrEP.
· Underscore importance of counseling about adherence and HIV risk reduction, including encouraging condom use for additional protection.
· Recommend regular monitoring of HIV infection status, side effects, adherence, and sexual or injection risk behaviors.
· Include a providers’ supplement with additional materials and tools for use when prescribing PrEP.
The new federal guidelines recommend that PrEP be considered for people who are HIV-negative and at substantial risk for HIV.
For sexual transmission, this includes anyone who is in an ongoing relationship with an HIV-positive partner. It also includes anyone who 1) is not in a mutually monogamous* relationship with a partner who recently tested HIV-negative, and 2) is a
· gay or bisexual man who has had anal sex without a condom or been diagnosed with an STD in the past 6 months; or
· heterosexual manor woman who does not regularly use condoms during sex with partners of unknown HIV status who are at substantial risk of HIV infection (e.g., people who inject drugs or have bisexual male partners).
For people who inject drugs, this includes those who have injected illicit drugs in the past 6 months and who have shared injection equipment or been in drug treatment for injection drug use in the past 6 months.
Health care providers should also discuss the use of PrEP with HIV discordant heterosexual couples (in which one partner is HIV-positive and the other HIV-negative) during conception and pregnancy as one of several options to protect the partner who is HIV-negative.
Patients on PrEP should return to their health care provider every 3 months for a repeat HIV test and other follow-up. At this time, the provider can write a prescription refill, offer counseling about medication adherence and risk reduction, test for STDs if necessary, and assess side effects.
PrEP is only for people who are at ongoing substantial risk of HIV infection. For people who need to prevent HIV after a single high-risk event of potential HIV exposure—such as unprotected sex, needle-sharing injection drug use, or sexual assault—there is another option called postexposure prophylaxis, or PEP. PEP must begin within 72 hours of exposure. See our PEP Q&A for more information.
PrEP Clinical Trials
The guidelines are based on strong evidence from clinical trials of PrEP use in high-risk populations. All participants in these trials received pills containing either PrEP or placebo (a pill without any medicine in it), along with intensive counseling on safe-sex behavior, regular testing for STDs, and a regular supply of condoms.
In all of these studies, HIV transmission risk was lowest for participants who took the pill consistently. Specifically:
· Among gay and bisexual men, those who were given PrEP were 44% less likely overall to get HIV than those who were given a placebo. Among the men with detectable levels of medicine in their blood (meaning they had taken the pill consistently), PrEP reduced the risk of infection by as much as 92%. (iPrEx Study)
· Among heterosexually active men and women, PrEP reduced the risk of getting HIV by 62%. Participants who became infected had far less drug in their blood, compared with matched participants who remained uninfected. (TDF2 Study)
· Among men and women in HIV discordant couples, those who received PrEP were 75% less likely to become infected than those on placebo. Among those with detectable levels of medicine in their blood, PrEP reduced the risk of HIV infection by up to 90%. (Partners PrEP Study)
· Among injection drug users, a once-daily tablet containing tenofovir (one of the two drugs prescribed) reduced the risk of getting HIV by 49%. For participants who had detectable tenofovir in their blood, PrEP reduced the risk of infection by 74%. (Bangkok Tenofovir Study)
None of the studies found any significant safety concerns with use of daily oral PrEP. Some trial participants reported side effects such as an upset stomach or loss of appetite but these were mild and usually resolved in the first month.
* Mutually monogamous means that you and your partner only have sex with each other and do not have sex outside the relationship.
|
|
| |
| |
|
|
|