| |
Dolutegravir & HIV Eradication Strategy
|
| |
| |
from Jules: in treatment-naive studies of dolutegravir (DTG) no integrate mutations have been found in patients with viral failure. a resistance mutation R263K has been found associated with failure to DTG but there is controversy whether the 263 reduces clinical effectiveness of DTG or not, Wainberg says it reduces replication capacity and DTG continues to be effective despite the 263, below you will see link to Euro Resist Wk where there is a report on 2 patients in SAILING, a study of patients receiving DTG but who had prior ART experience & 2 patients developed the 155 but a lot of data shows DTG is active against 155, but DTG failure appears associated with non-adherence. The theory by Mark Wainberg is that dolutegravir can play a role in providing a functional cure, read through review by Mark Wainberg just published in the journal Viruses.....
What if HIV were unable to develop resistance against a new therapeutic agent? http://www.natap.org/2015/HIV/071415_02.htm ".....what will happen if patients do as well on DTG monotherapy as on triple therapy, despite the presence of the R263K mutation. Would clinicians then be willing to entertain the notion of withholding DTG from therapy at a certain point as part of a structured treatment interruption? In this scenario, it is conceivable that the impaired viruses containing DTG resistance mutations would not be able to grow out. What would then become of the wild-type viruses that had become archived after infecting the patient in the first place? Presumably, a high proportion of such viruses would begin to replicate following activation of latent reservoirs in the same manner as has been observed following treatment interruption in other trials. However, re-initiation of DTG monotherapy might then convert these wild-type viruses into DTG-resistant attenuated forms. Is it conceivable that a number of cycles of DTG treatment interruption followed by re-initiation of DTG monotherapy could convert all the HIV in the body to a replication impaired form? Could such an approach lead to a functional cure of HIV disease if all residual viruses were significantly impaired in viral replication and if further compensatory mutations were unable to develop?"
In this study at IAS/Vancouver in 1 patient who developed 263 resistance evolved to include 138 & 147 and 5-fold reduced DTG susceptibility BUT Wainberg says "is this a very rare, anecdotal case of a non-adherent person is a typical one ......This anecdotal case is similar to the SAILING patients who developed R263K, because some of them were on effective DTG mono therapy.....2 years after the approval of DTG by the FDA and hence its real-world use in such settings as inner city Washington and the Bronx, that there has not been a single case described from such settings and in clinical trials of resistance to either DTG or the NRTIs with which it has been co-utilized.....It is also notable that this patient's viral load did not skyrocket if this was a true case of resistance. Rather, it is proof that DTG continues to be active despite the presence of the R263K mutation" http://www.natap.org/2015/IAS/IAS_32.htm.......Resistance mutations against dolutegravir in HIV integrase impair the emergence of resistance against reverse transcriptase inhibitors: "R263K was later detected in two highly treatment-experienced patients who failed DTG in a trial termed SAILING"......http://www.natap.org/2015/HIV/071415_01.htm....."Our data are consistent with the notion that R263K may not be a deleterious mutation for DTG"......"We conclude that the presence of DTG when used together with other active drugs may not lead to virological failure even if the R263K substitution is present. This hypothesis could be tested by ultrasensitive sequencing of integrase from residual plasma viral RNA in individuals who have been successfully treated with DTG or from the DNA of lymphocytes of such individuals."
------------------------------
Dolutegravir Functional Eradication Strategy......
http://www.mdpi.com/1999-4915/7/7/2790/htm
"It is now almost two years since the approval of DTG by the US Food and Drug Administration (FDA), with no cases of clinical resistance to DTG having yet been reported in the literature in the aftermath of first-time DTG use outside the context of defined clinical trials. Nonetheless, we must be wary that resistance to DTG in such settings may ultimately occur."
".....we believe that DTG may prevent residual viral replication-competent viruses from becoming part of the viral reservoir.....".......this hypothesis is currently being evaluated through a registered clinical trial in individuals initiating therapy with DTG as soon as possible after HIV diagnosis.....https://clinicaltrials.gov/ct2/show/NCT02370979?term=NCT02370979&rank=1.......Importantly, stable, non-replicating reservoirs will not be affected by treatment intensification with DTG. However, other clinical trials that will include measurements of the size of HIV reservoirs in response to treatment intensification with DTG may be initiated and such trials might have a more positive outcome than those that were performed with RAL. Negative results on the size of the viral reservoir were also reported when both RAL and the CCR5 antagonist maraviroc were used to intensify antiretroviral therapy [68,69,70]. Importantly, the M184I substitution was transiently detected within the HIV integrated DNA from one individual undergoing RAL intensification [71], an observation that confirmed that RAL use does not protect against the emergence of viruses that are resistant against NRTIs. Together with the observation that optimal dose of DTG does protect against the emergence of such viruses, even in individuals failing DTG-based therapy (Table 3), this supports the hypothesis of potential added benefit with DTG intensification........the reintroduction of DTG as part of a second round of therapy after treatment interruption and/or treatment failure should lead to re-suppression of viral load and, as well, this should result in the killing of both the viruses that may have become activated from reservoirs of long-lived latently infected T-cells as well as actively replicating viruses, regardless whether such viruses are WT or contain mutations associated with drug resistance against any type of ARV. Since the numbers of cells in the body that are capable of serving as hosts of the HIV reservoir must be finitely limited, the use of a drug against which resistance might not be able to occur could change the paradigm of HIV therapeutics and help to point the way forward to a successful eradication strategy.
In SINGLE vs EFV 39 patients on DTG failed with no DTG or integrate resistance found.......
http://www.natap.org/2014/ICAAC/ICAAC_11.htm
In SPRING-1 a phase 2b study with multi-doses of DTG (10,25, 50 mg) vs EFV, 155 patients recd one of these 3 doses with 3 protocol-defined viral failures & no integrate resistance found.......
http://www.natap.org/2012/CROI/croi_22.htm... SPRIN-2 in treatment-naives compared DTG full-dose 50 mg to raltegravir, there were 22 failures on DTG, no integrate mutations found .....
http://www.natap.org/2013/IAS/IAS_50.htm
FLAMINGO in treatment compared DTG to Darunavir, 2 patients failed DTG, neither with integrate resistance .......
http://www.natap.org/2013/ICAAC/ICAAC_24.htm
SAILING ...in treatment-experienced patients with NRTI-resistance but no integrate experience, DTG compared to raltegravir......
http://www.natap.org/2014/IAC/IAC_20.htm
Package Insert:
Treatment-naïve Subjects: No subjects in the dolutegravir 50-mg once-daily treatment arms of treatment-naïve trials SPRING-2 and SINGLE had a detectable decrease in susceptibility to dolutegravir or background NRTIs in the resistance analysis subset (n = 9) with HIV-1 RNA greater than 400 copies per mL at failure or last visit through Week 96 and having resistance data. One subject in SINGLE with 275 copies per mL HIV-1 RNA had a treatment-emergent integrase substitution (E157Q/P) detected at Week 24, but no corresponding decrease in dolutegravir susceptibility. No treatment-emergent genotypic resistance to the background regimen was observed in the dolutegravir arm in either the SPRING-2 or SINGLE trials. No treatment-emergent primary resistance substitutions were observed in either treatment group in the FLAMINGO trial.
Euro Resistance Wk: Resistance Post Week 48 in ART-Experienced, Integrase Inhibitor-Naive Subjects With Dolutegravir (DTG) vs. Raltegravir (RAL) in SAILING (ING111762)......
http://www.natap.org/2015/HIV/061715_02.htm [from Jules: in 2 subjects from SAILING the 155 mutation did emerge but it appears they were still sensitive to DTG
The Activity of the Integrase Inhibitor Dolutegravir Against HIV-1 Variants (155) Isolated From Raltegravir-Treated Adults...........
http://www.natap.org/2012/HIV/110812_01.htm
Using clinically derived samples, we report that dolutegravir often retains full or near-full activity against variants that possess genotypic and phenotypic resistance to raltegravir. This is particularly true for isolates containing common raltegravir-associated mutations at positions 143 and 155 in the integrase open-reading frame......In vitro data have demonstrated that dolutegravir retains substantial activity against Y143 and N155H pathway virus with additional secondary mutations and against virus with Q148 mutations alone.5 Dolutegravir activity has a broader range of FC resistance against Q148 pathway virus with additional raltegravir secondary mutations; resistance generally increases with increasing number of mutations.
Resistance to HIV Integrase Strand Transfer Inhibitors Among Clinical Specimens in the United States, 2009-2012; Dolutegravir Resistance Reports......
http://www.natap.org/2014/HIV/012214_03.htm
Antiviral Activity of Dolutegrevir in Subjects With Failure on an Integrase Inhibitor-Based Regimen: Week 24 Phase 3 Results From VIKING-3 (1) Poster.......
http://www.natap.org/2012/interHIV/InterHIV_05.htm
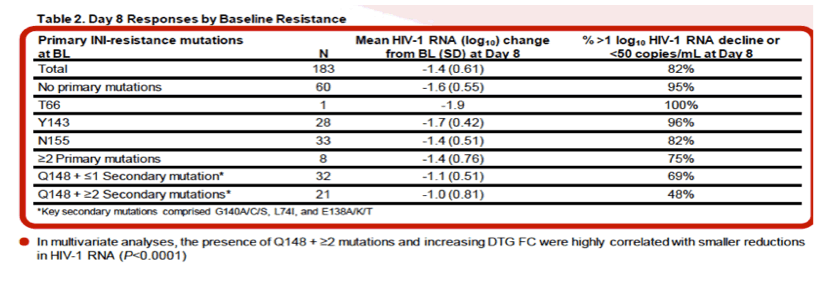
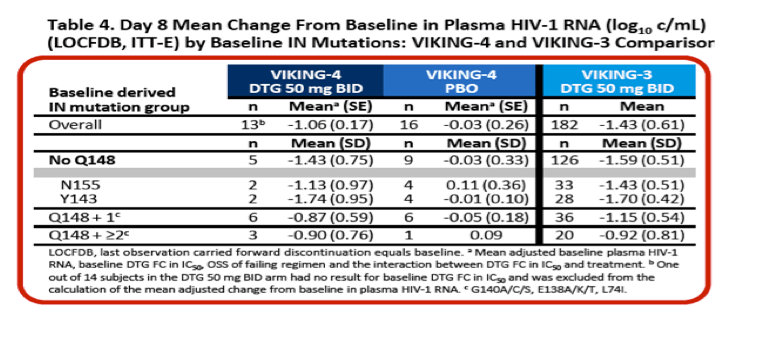
Activity of Dolutegravir (DTG) 50 mg BID vs Placebo (PBO) Over 7 Days of Functional Monotherapy in Patients Harbouring Raltegravir- and/or Elvitegravir-Resistant Virus: Primary Endpoint Results of the VIKING-4 Study (ING116529)......14th European AIDS Conference Oct 16-19 2013 Brussels, Belgium......
http://www.natap.org/2013/EACS/EACS_01.htm
|
|
| |
| |
|
|
|