| |
Longitudinal Changes Over 10 Years in Free Testosterone
Among HIV-Infected and HIV-Uninfected Men
|
| |
| |
Download the PDF here
"To our knowledge, this is the largest and longest study, which has compared longitudinal changes in gonadal function among HIV-infected and HIV-uninfected men. We found that FT decreased similarly over a 10-year interval in both groups. However, AM FT levels were lower among HIV-infected men compared with the HIV-uninfected men, but not PM levels. These findings may suggest a loss of diurnal variation in FT among HIV-infected men, a commonly recognized phenomenon among aging men.......In conclusion, although HIV-infected and HIV-uninfected men experienced similar declines in FT over the 10-year study interval, FT was lower and had smaller diurnal variation among the HIV-infected men. Taken together, our findings may suggest a premature aging of the HPG axis in HIV-infected men based on blunting of the normal diurnal variation, but further studies are needed to test this hypothesis. [from jules: the authors suggest immune activation & inflammation might adversely affect FT but in this study analysis did not find that, and "In the general population, the decline in gonadal function with aging has been associated with multiple adverse health outcomes, including cardiovascular diseases, DM, osteoporosis, bone fractures, and metabolic syndrome,1,2 although it is unclear if these relationships are causal", but in this study there did not I think appear to be an association although HIV+ had more comorbidities.]
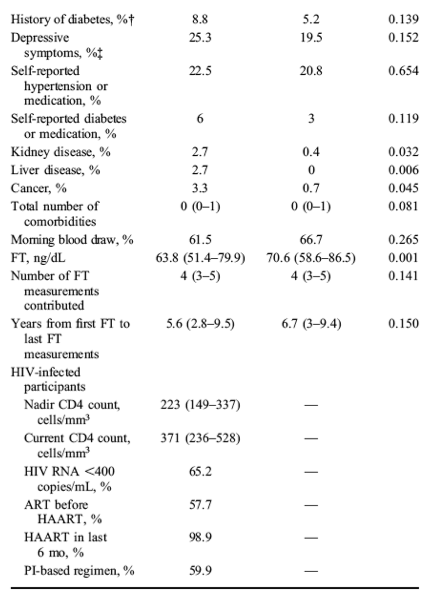
The significant declines in FT by age did not differ by HIV serostatus (P = 0.58); mean FT decreases per 10-year increase in age were 9.2 ng/dL [95% confidence interval (CI): -13.4 to -5.0] and 7.9 ng/dL (95% CI: -10.2 to -5.5) among HIV-infected and HIV-uninfected men, respectively. There was no difference in overall FT level by HIV serostatus [-3.5 ng/dL (95% CI: -8.0 to 0.98), P = 0.12]. Mean FT levels measured from samples drawn in the AM were higher than levels measured in the PM [4.9 ng/dL (95% CI: 2.4 to 7.4), P = 0.0001] (Fig. 1A).......The HIV-infected men were older, had a lower BMI, and were more likely than the HIV-uninfected men to be current smokers and HCV-infected (Table 1). The prevalence of DM and the racial distribution were similar among groups. The comorbidity score was higher in the HIV-infected men.
In a second model, the interaction of HIV serostatus x time of blood draw (AM vs. PM) approached statistical significance (P = 0.06), providing some evidence that the relationship between HIV serostatus and FT depended on the time of the blood draw (Fig. 1B). In this model, HIV-infected men had lower FT than HIV-uninfected men [-5.0 ng/dL (95% CI: -9.8 to -0.29) at age 45, P = 0.037]. The adjusted mean FT level in AM samples was significantly lower among HIV-infected compared with HIV-uninfected men [-6.1 ng/dL (95% CI: -9.8 to -2.4), P = 0.001], but a significant difference was not observed in the PM samples [-1.7 ng/dL (95% CI: -6.0 to -2.6), P = 0.441]. The AM and PM FT levels did not differ significantly among the HIV-infected men [73.3 ng/dL (95% CI: 69.3 to 77.2) vs. 70.9 ng/dL (95% CI: 66.5 to 75.3), P = 0.193], but, among the HIV-uninfected men, the AM levels of FT were significantly higher [78.3 ng/dL (95% CI: 75.6 to 80.9) vs. 71.5 ng/dL (95% CI: 68.3 to 74.7), P < 0.001]. Similar to the previous model, the rate of FT decline with advancing age in this model, which included an HIV serostatus x time of blood draw interaction term did not differ significantly by HIV serostatus. In an exploratory analysis, the addition of comorbidity score to the fully adjusted model did not appreciably change our results (data not shown).
Our finding that FT decreases at a similar rate among HIV-infected and HIV-uninfected men suggests that andropause is not accelerated among ART-treated HIV-infected men. It should be noted, however, that our analysis excluded men who reported testosterone use or had FT levels in the super physiological range, suggestive of exogenous testosterone use. Since these men may have more pronounced HPG dysfunction and such men are more likely to be HIV-infected,11 our analysis may have underestimated the differences in the longitudinal changes in FT between HIV-infected and HIV-uninfected men. In addition, the age distribution of studied men was relatively narrow. Longer-term studies are required to better understand the changes in HPG function among HIV-infected men in their seventh and eighth decade.
Although changes in FT over the study interval were similar by HIV serostatus, HIV-infected men on average had lower FT compared with HIV-uninfected men. These results are consistent with our previous cross-sectional substudy in the MACS in which FT was significantly lower among HIV-infected than HIV-uninfected men.11Other cross-sectional studies of HIV-infected persons have shown similar findings, but most were performed in the early pre-HAART era, examined HIV-infected persons with wasting, or did not include an internal HIV-uninfected comparison group.6,10,11,30
Differences in FT by HIV serostatus in our study were apparent only for FT samples collected in the AM rather than the PM. In men with a normal sleep/wake cycle, there is diurnal variation of testosterone secretion with a peak between 6 and 9 AM with nadir levels at approximately 6 PM.31-33 The magnitude of the diurnal variation decreases with age. For example, in a study which measured FT every 30 minutes for 24 hours in 10 young (23-33 years) and 8 older (55-64 years) men, diurnal variation was approximately 40% in the younger men and 30% in the older men.34 Our finding that FT differed by time of blood draw in the HIV-uninfected but not the HIV-infected men may suggest that the AM rise in testosterone is blunted in men with HIV infection and the diurnal variation is decreased, similar to the effect of normal aging. Prospective studies with multiple blood draws over the course of the day among HIV-infected men and matched controls are needed to test this hypothesis.
One potential mechanism for the loss of diurnal variation with aging is the effect of systemic inflammation on gonadal function. Markers of systemic inflammation increase with aging35 and have been shown to be inversely correlated with serum testosterone. Although it has been hypothesized that lower testosterone production may lead to systemic inflammation,36 another possible explanation is that systemic inflammation directly affects gonadal function. Administration of IL-6 or TNF to healthy male volunteers has been shown to decrease testosterone in a dose-dependent manner, suggesting a direct effect on gonadal function.37,38 In a preclinical model, TNF infusion also inhibited the secretion of gonadotropin releasing hormone (GnRH).39 More recently, the activation of the IKK-ß/NFkB pathway in the hypothalamus of mice led to a decline of GnRH secretion. Thus, systemic immune activation may also have detrimental effects on GnRH secretion.19 In our study, the inclusion of biomarkers of systemic inflammation/immune activation in the statistical models did not attenuate the difference in AM FT by HIV serostatus or the effect of aging on FT in our regression models. Therefore, inflammation or immune activation did not explain the differences in FT between the HIV-infected and HIV-uninfected men.
The clinical impact of lower FT among HIV-infected men is unclear. The effect of HIV serostatus in our regression models was equivalent to approximately 6 years of aging. In the general population, the decline in gonadal function with aging has been associated with multiple adverse health outcomes, including cardiovascular diseases, DM, osteoporosis, bone fractures, and metabolic syndrome,1,2 although it is unclear if these relationships are causal.21,23-25 Ongoing clinical trials of testosterone replacement in older men will help answer this important question.40"
----------------------------
Longitudinal Changes Over 10 Years in Free Testosterone Among HIV-Infected and HIV-Uninfected Men
JAIDS Jan 12016
Slama, Laurence MD*,; Jacobson, Lisa P. ScD, ScM; Li, Xiuhong MAS; Palella, Frank J. Jr MD; Margolick, Joseph B. MD; Kingsley, Lawrence A. PhD; Wiley, Dorothy J. PhD; Pialoux, Gilles MD*; Dobs, Adrian S. MD#; Brown, Todd T. MD, PhD#; for the Multicenter AIDS Cohort Study (MACS)
*Division of Infectious Diseases, Tenon Hospital, Pierre and Marie Curie University, Paris, France; Division of Infectious Diseases, Feinberg School of Medicine, Northwestern University, Chicago, IL; Departments of Epidemiology; Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD; Department of Infectious Diseases and Microbiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA; Division of Translational Science, School of Nursing, UCLA, Los Angeles, CA; and #Division of Endocrinology, Diabetes and Metabolism, Johns Hopkins University School of Medicine, Baltimore, MD.
Abstract
Background: Aging in males is associated with lower testosterone levels and a decrease in diurnal variation of testosterone secretion. Cross-sectional studies have shown lower than expected testosterone levels among HIV-infected men, but whether age-related changes in serum testosterone differ by HIV serostatus are not known.
Methods: HIV-infected men from the Multicenter AIDS Cohort Study (MACS), age ≥45 years at highly active antiretroviral therapy initiation, who had ≥2 samples from the subsequent 10 years, were matched to HIV-uninfected men by age, race, MACS site, and calendar time of samples. Linear mixed-effects regression models were used to determine whether free testosterone (FT) and its rate of change differed by HIV serostatus.
Results: One hundred eighty-two HIV-infected and 267 HIV-uninfected men were included, median age: 48.8 years (interquartile range: 45.8-53.4), median numbers of FT measurements per participant 4 (interquartile range: 3-5), 65% were drawn in the adjusted morning (AM). Mean-adjusted FT levels were lower among HIV-infected than HIV-uninfected men in AM samples {-6.1 ng/dL [95% confidence interval (CI): -9.8 to -2.4], P = 0.001}, but not in afternoon samples [-1.7 ng/dL (-6.0 to 2.6), P = 0.441]. The rate of FT decline with age did not differ by HIV serostatus: 9.2 ng/dL (95% CI: -13.4 to -5.0) per 10 years for HIV-infected vs. 7.9 ng/dL (95% CI: -10.2 to -5.5) for HIV-uninfected men, P = 0.578.
Conclusions: FT decreased similarly with increasing age regardless of HIV serostatus. The lower AM, but not adjusted afternoon, FT levels among HIV-infected men compared with HIV-uninfected men suggest a loss of diurnal variation in FT levels among HIV-infected men.
INTRODUCTION
In the general male population, testosterone levels decrease with age and may contribute to age-related comorbidities, including sexual dysfunction, sarcopenia, osteoporosis, glucose abnormalities, and cardiovascular disease.1,2 In the Third National Health and Nutrition Examination Survey, 12.8% of men between 50 and 59 years had total testosterone (TT) levels in the hypogonadal range using a cutoff of 300 ng/dL. In men over 70 years, the prevalence of hypogonadism was 24.9%. Age-related changes in the gonadal axis are even more pronounced if free testosterone (FT) levels are examined rather than TT as sex hormone binding globulin (SHBG) increases with aging. More than 30% of men over 70 years have FT concentrations in the hypogonadal range (<4.9 ng/dL).3-5
Hypogonadism has been a commonly recognized condition among HIV-infected men since early in the HIV epidemic with consequences on fat and lean total body mass, muscle strength, bone mineral density, and physical function. With effective antiretroviral therapy, TT and FT levels increase,6 but hypogonadism remains a common problem among HIV-infected men with prevalence estimates ranging from 21% to 70%.4,7-10 In a previous cross-sectional study conducted in the Multicenter AIDS Cohort Study (MACS) during the era of highly active antiretroviral therapy (HAART),11 we found that hypogonadism (defined as a level of FT or TT below the lower limit of normal or use of testosterone replacement therapy) was more common in HIV-infected men compared with HIV-uninfected participants (24.5% vs. 7.8%). Among those not receiving or reporting testosterone use, the lower adjusted FT concentrations among HIV-infected men were equivalent to 13 years of aging.12,13 Although FT levels decreased with increasing age in this study, the magnitude of decrease was similar by HIV serostatus, and no interaction between HIV serostatus and age was observed.
There is limited published data on the longitudinal changes in TT or FT levels among older HIV-infected men compared with otherwise similar HIV-uninfected men. We undertook a longitudinal, nested cohort study with in the MACS to determine whether age-related changes in FT differed by HIV serostatus.
METHODS
Study Population
The MACS is a prospective study of men who have sex with men who are HIV-infected or at risk for HIV-1 infection, ongoing since 1984 at 4 US sites: Chicago, Baltimore/Washington DC, Pittsburgh, and Los Angeles. Details of the study design and methods have been published.14 The institutional review boards of each site approved the study protocols, and informed consent was obtained from each participant.
Selection Criteria
We identified HIV-infected men who were at least 45-year old at HAART initiation, with at least 2 samples available from the 10 years after HAART initiation. These men were matched to HIV-uninfected men by age (±5 years), race, MACS site, and calendar time of sample collection. Men who reported taking exogenous hormones of any kind and/or had FT concentrations >150 ng/dL suggestive of unreported testosterone use were excluded from the analysis.
Laboratory Methods
All hormone assays were performed using frozen samples in the laboratory of Dr Shalender Bhasin (Boston University, Boston). TT levels were measured from archived serum using liquid chromatographic-tandem mass spectrometrySHBG was measured using radioimmunoassay. FT was calculated from TT and SHBG measurement using the Vermeulen equation.15 For the exploratory analysis described below, serum levels of chemokines and proinflammatory cytokines [IL-1ß, IL-2, IL-6, IL-10, IL-12p70, IFN-γ, tumor necrosis factor (TNF)-α, Eotaxin, IL-8/CXCL8, IP-10/CXCL10, MCP-1/CCL2, MCP-4/CCL13, MIP-1ß/CCL4, hsCRP, and TARC/CCL17] were assessed using the Meso-Scale Discovery (MSD) Multi-Array platform (Meso-Scale Diagnostics, LLC, Rockville, MD) in the laboratory of Dr. Jay Bream (Johns Hopkins Bloomberg School of Public Health, Baltimore, MD). Sensitivities for cytokines were typically in the range of 1 pg/mL, whereas chemokine detection ranged from 39 to 158 pg/mL. Serum levels of the soluble receptor markers were determined using the multiplexed Luminex xMAP system (Fluorokine MAP, R&D systems, Minneapolis, MN). Levels of 5 soluble receptors (sCD14, sgp130, sIL-2Rα, sTNFR2, sIL6R), plus a cytokine (BAFF/BLyS), and the chemokine BLC-BCA1/CXCL13 were measured in one panel (Human Biomarker Custom Premix Kit A). Testing of all biomarkers was conducted in the laboratory of Dr Oto Martinez-Maza (University of California, Los Angeles, CA). Results for these biomarkers of inflammation and immune activation in a larger MACS cohort have been previously reported.16 Serum samples for biomarker measurement were chosen from semiannual MACS visits closest to the FT study visits (±1 year).
Other Covariates
Variables of interest included race [black, Hispanic, and white (reference)], hepatitis C virus (HCV) serostatus (defined by positive antibody or positive HCV-RNA, Yes/No), body mass index [BMI: <20, 20-24.9 (reference), 25-29.9, ≥30 kg/m2], tobacco smoking [current, past, and never (reference)], history of diabetes mellitus [DM: fasting glucose ≥126 mg/dL or self-reported DM and use of DM medications versus no DM (reference)], time of blood draw for FT measurement (morning or afternoon/evening), and cumulative years on HAART for HIV-infected men assessed from data collected at semiannual visits.
Statistical Analysis
The first post-HAART visit with a serum sample available for FT testing was defined as the baseline visit. Baseline characteristics among HIV-infected and HIV-uninfected men were compared using nonparametric Wilcoxon test or χ2 or Fisher exact test as appropriate. A linear mixed-effects regression model with t-distributed error (degrees of freedom were estimated in models) was used to determine whether FT and its rate of change with increasing age differed by HIV serostatus. A random intercept assumed to be normally distributed was included in the mixed model.
Factors in the first multivariable linear mixed model included HIV serostatus, age, race [white (reference), black, and Hispanic], study center, smoking status, history of DM, BMI, HCV status and time of blood draw for FT measurement [morning (AM) vs. afternoon (PM)], and the interaction term of HIV serostatus · age. In a second model, the interaction of HIV serostatus · time of blood draw (AM vs. PM) was added.
We performed 2 exploratory analyses to better understand our results. First, since there is a debate as to whether the decrease in gonadal function with increasing age is a result of the accumulation of age-related comorbid conditions, we adjusted the linear mixed model to include a comorbidity score, which was defined as the total count of the following 6 comorbid conditions: (1) depression (Centers for Epidemiologic Studies Depression Scale score >16);17,18 (2) self-reported hypertension or receiving antihypertension medications; (3) self-reported DM or receiving diabetes medication; (4) kidney disease (estimated glomerular filtration rate less than 60 mL/min per 1.73 m2 using the Modification of Diet in Renal Disease equation or a urine protein-to-creatinine ratio greater than or equal to 200 mg protein/1 g creatinine, or confirmed kidney disease diagnosis with ICD-9 codes 580-591 and 593); (5) liver disease (alanine transaminase or aspartate aminotransferase >150 IU/L, or confirmed liver disease diagnosis with ICD-9 codes 570-573 excluding hepatitis) and; (6) Cancer (diagnosis at or within a year before the relevant visit). History of diabetes was excluded from the model that included the comorbidity score. Also, since increased systemic inflammation has been proposed as a mechanism by which age-related decreases in gonadal function occur and is associated with HIV infection itself,19 we sought to determine whether levels of biomarkers of inflammation and immune activation, using the chemokines and cytokines listed above, were associated with FT levels and whether inclusion of these biomarkers in multivariable models impacted the effects of HIV infection or time of blood draw (AM vs. PM) on FT levels. We evaluated biomarker levels by HIV serostatus using generalized gamma regression models adjusted for age, race, and BMI. We then added each biomarker to the fully adjusted longitudinal mixed model that already included an HIV serostatus x time of blood draw interaction term and the comorbidity score described above to examine individual biomarker associations with FT and on point estimates for HIV serostatus, age, and time of blood draw associations with FT. The biomarkers were log2 transformed such that the coefficients represent the effect per doubling of biomarker level. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
Between October 1, 1995, and September 30, 2009, there were 306 HIV-infected men who met study inclusion criteria. Of those, 271 were matched with 271 HIV-uninfected men. Eighty-nine HIV-infected men and 4 HIV-uninfected men were excluded from the analysis because they were found to have an excessively high level of FT (>150 ng/dL) at ≥ 1 visit (ie, >99 percentile of FT in the HIV-uninfected men). Because these levels are unlikely to be physiologically plausible, we assumed that these participants were using exogenous testosterone but did not report this administration during their MACS visits. Most of these men (60%) were from the Los Angeles site.
At the baseline visit, our analytic data set included 449 men (182 HIV-infected and 267 HIV-uninfected men). The HIV-infected men were older, had a lower BMI, and were more likely than the HIV-uninfected men to be current smokers and HCV-infected (Table 1). The prevalence of DM and the racial distribution were similar among groups. The comorbidity score was higher in the HIV-infected men. At the baseline visit, 99% of the HIV-infected participants were HAART treated (60% with a PI-containing regimen and 37% with a NNRTI-containing regimen) and 58% had received antiretroviral treatment (ART) before HAART initiation. The median CD4 cell count was 371 cells per cubic millimeter, and 65% of HIV-infected men had plasma HIV RNA <400 copies per milliliter. During the study interval, 267 HIV-uninfected men contributed 1051 person-visits, and 182 HIV-infected men contributed 686 person-visits, 72% of which had an associated HIV RNA levels <400 copies per milliliter. The median number of FT measurements per participant was 4 (interquartile range: 3-5) drawn over a median of 6 years (interquartile range; 2.9-9.5). Of these, 65% were drawn in the AM.
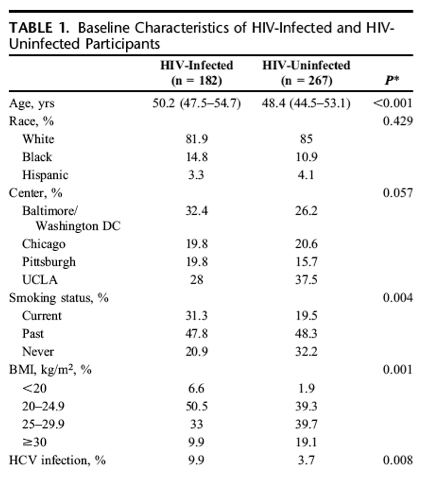
Longitudinal Changes in Free Testosterone by HIV-Status
The significant declines in FT by age did not differ by HIV serostatus (P = 0.58); mean FT decreases per 10-year increase in age were 9.2 ng/dL [95% confidence interval (CI): -13.4 to -5.0] and 7.9 ng/dL (95% CI: -10.2 to -5.5) among HIV-infected and HIV-uninfected men, respectively. There was no difference in overall FT level by HIV serostatus [-3.5 ng/dL (95% CI: -8.0 to 0.98), P = 0.12]. Mean FT levels measured from samples drawn in the AM were higher than levels measured in the PM [4.9 ng/dL (95% CI: 2.4 to 7.4), P = 0.0001] (Fig. 1A).
In a second model, the interaction of HIV serostatus x time of blood draw (AM vs. PM) approached statistical significance (P = 0.06), providing some evidence that the relationship between HIV serostatus and FT depended on the time of the blood draw (Fig. 1B). In this model, HIV-infected men had lower FT than HIV-uninfected men [-5.0 ng/dL (95% CI: -9.8 to -0.29) at age 45, P = 0.037]. The adjusted mean FT level in AM samples was significantly lower among HIV-infected compared with HIV-uninfected men [-6.1 ng/dL (95% CI: -9.8 to -2.4), P = 0.001], but a significant difference was not observed in the PM samples [-1.7 ng/dL (95% CI: -6.0 to -2.6), P = 0.441]. The AM and PM FT levels did not differ significantly among the HIV-infected men [73.3 ng/dL (95% CI: 69.3 to 77.2) vs. 70.9 ng/dL (95% CI: 66.5 to 75.3), P = 0.193], but, among the HIV-uninfected men, the AM levels of FT were significantly higher [78.3 ng/dL (95% CI: 75.6 to 80.9) vs. 71.5 ng/dL (95% CI: 68.3 to 74.7), P < 0.001]. Similar to the previous model, the rate of FT decline with advancing age in this model, which included an HIV serostatus x time of blood draw interaction term did not differ significantly by HIV serostatus. In an exploratory analysis, the addition of comorbidity score to the fully adjusted model did not appreciably change our results (data not shown).
EXPLORATORY ANALYSES: EFFECT OF INFLAMMATORY BIOMARKERS ON FT
Of 449 men included in the primary analysis, 224 men (715 person-visits) with both FT and serum biomarker measurements available were included in the analysis of the association between biomarkers and FT (Table 2). At the baseline visit, men with biomarkers were more likely to be older, non-white, HIV-infected, HCV-infected, and current smokers compared with men for whom biomarkers levels were not available.
After adjusting for age, race, and BMI, levels of multiple biomarkers were significantly associated with HIV infection: BAFF [relative percentile (RP)] HIV-infected vs. HIV-uninfected 1.17 (95% CI: 1.07 to 1.29), sCD14 [RP = 1.26 (95% CI: 1.16 to 1.38)], sCD27 [RP = 1.33 (95% CI: 1.20 to 1.48)], sIL-2Rα [RP = 1.12 (95% CI: 1.02 to 1.24)], sTNFR2 [RP = 1.24 (95% CI: 1.23 to 1.38)], TNF-α [RP = 1.17 (95% CI: 1.05 to 1.30)], IP-10 [RP = 1.67 (95% CI: 1.41 to 2.00)], MCP-4 [RP = 1.25 (95% CI: 1.08 to 1.46)], MCP-1 (RP = 1.22 (95% CI: 1.08 to 1.38)], hsCRP [RP = 1.53 (95% CI: 1.08 to 2.13)], IL-10 [RP = 0.79 (95% CI: 0.63 to 0.98)], IL-12 p70 [RP = 0.68 (95% CI: 0.45 to 0.97)], and GM-CSF [RP = 0.68 (95% CI: 0.49 to 0.98)]. No differences by HIV serostatus were found for levels of BLC-BCA1, gp130, sIL6R, IL-6, IL-8, eotaxin, MIP-1ß, TARC, IFN-γ, IL1B, and IL-2.
We then evaluated whether: (1) these biomarkers were associated with FT and (2) whether the inclusion of each biomarker in the fully adjusted regression model affected the point estimates assessing the relationship among HIV serostatus, age, timing of blood draw, and FT. Of the biomarkers examined, gp130, MCP-1, sCD14, sTNFR2, BAFF, IL-6, TARC, and hsCRP levels were each significantly associated with lower FT (P < 0.05, for each) (Table 3). When each of these markers was added individually to the regression model, the effect estimates for HIV serostatus stratified by time of blood draw or age stratified by HIV serostatus did not change appreciably. For example, at age 52 years, FT level drawn in the AM was 10.3 ng/dL lower among HIV-infected than HIV-uninfected men (Column 1, Table 3). After the inclusion of gp130 in the same statistical model, the effect of HIV serostatus on AM FT was very close to the previous model (-9.8 ng/dL). Similarly, the effects of age on FT among both HIV-infected and HIV-uninfected men did not change with the addition of gp130 or any of the other biomarkers that were associated with lower FT into the regression model.
DISCUSSION
To our knowledge, this is the largest and longest study, which has compared longitudinal changes in gonadal function among HIV-infected and HIV-uninfected men. We found that FT decreased similarly over a 10-year interval in both groups. However, AM FT levels were lower among HIV-infected men compared with the HIV-uninfected men, but not PM levels. These findings may suggest a loss of diurnal variation in FT among HIV-infected men, a commonly recognized phenomenon among aging men.
In the general male population, multiple cross-sectional and longitudinal studies have shown that gonadal function decreases with age, an occurrence that has been termed "andropause."20-26 For example, in the 10-year follow-up Massachusetts Male Aging study,22 a large cross-sectional study of men 39-70 year old, each additional year of age was associated with an approximate 1.2% decrease in FT. These cross-sectional results have been confirmed in longitudinal studies.20,22,26This study found very similar results: FT declined at approximately 1.2% per year in both HIV-infected and HIV-uninfected men. This result is also consistent with our previous cross-sectional study in the MACS.11
Mechanisms underlying reductions in testosterone with aging are not entirely clear. Both testicular dysfunction and inadequate gonadotropin production have been implicated.27,28 It has also been suggested that the decrease in testosterone among older men may represent aggregative long-term effects of multiple age-related comorbid conditions on the hypothalamic-pituitary-gonadal (HPG) axis, rather than aging per se.11 However, our data argue against this explanation because when we adjusted for multiple comorbidities,29 including smoking status, HCV-coinfection, history of diabetes, cumulative years on HAART for HIV-infected participants, depression, hypertension, kidney disease, liver disease, and cancer within a year, the effect of age on changes in FT was unaltered.
Our finding that FT decreases at a similar rate among HIV-infected and HIV-uninfected men suggests that andropause is not accelerated among ART-treated HIV-infected men. It should be noted, however, that our analysis excluded men who reported testosterone use or had FT levels in the super physiological range, suggestive of exogenous testosterone use. Since these men may have more pronounced HPG dysfunction and such men are more likely to be HIV-infected,11 our analysis may have underestimated the differences in the longitudinal changes in FT between HIV-infected and HIV-uninfected men. In addition, the age distribution of studied men was relatively narrow. Longer-term studies are required to better understand the changes in HPG function among HIV-infected men in their seventh and eighth decade.
Although changes in FT over the study interval were similar by HIV serostatus, HIV-infected men on average had lower FT compared with HIV-uninfected men. These results are consistent with our previous cross-sectional substudy in the MACS in which FT was significantly lower among HIV-infected than HIV-uninfected men.11Other cross-sectional studies of HIV-infected persons have shown similar findings, but most were performed in the early pre-HAART era, examined HIV-infected persons with wasting, or did not include an internal HIV-uninfected comparison group.6,10,11,30
Differences in FT by HIV serostatus in our study were apparent only for FT samples collected in the AM rather than the PM. In men with a normal sleep/wake cycle, there is diurnal variation of testosterone secretion with a peak between 6 and 9 AM with nadir levels at approximately 6 PM.31-33 The magnitude of the diurnal variation decreases with age. For example, in a study which measured FT every 30 minutes for 24 hours in 10 young (23-33 years) and 8 older (55-64 years) men, diurnal variation was approximately 40% in the younger men and 30% in the older men.34 Our finding that FT differed by time of blood draw in the HIV-uninfected but not the HIV-infected men may suggest that the AM rise in testosterone is blunted in men with HIV infection and the diurnal variation is decreased, similar to the effect of normal aging. Prospective studies with multiple blood draws over the course of the day among HIV-infected men and matched controls are needed to test this hypothesis.
One potential mechanism for the loss of diurnal variation with aging is the effect of systemic inflammation on gonadal function. Markers of systemic inflammation increase with aging35 and have been shown to be inversely correlated with serum testosterone. Although it has been hypothesized that lower testosterone production may lead to systemic inflammation,36 another possible explanation is that systemic inflammation directly affects gonadal function. Administration of IL-6 or TNF to healthy male volunteers has been shown to decrease testosterone in a dose-dependent manner, suggesting a direct effect on gonadal function.37,38 In a preclinical model, TNF infusion also inhibited the secretion of gonadotropin releasing hormone (GnRH).39 More recently, the activation of the IKK-ß/NFkB pathway in the hypothalamus of mice led to a decline of GnRH secretion. Thus, systemic immune activation may also have detrimental effects on GnRH secretion.19 In our study, the inclusion of biomarkers of systemic inflammation/immune activation in the statistical models did not attenuate the difference in AM FT by HIV serostatus or the effect of aging on FT in our regression models. Therefore, inflammation or immune activation did not explain the differences in FT between the HIV-infected and HIV-uninfected men.
The clinical impact of lower FT among HIV-infected men is unclear. The effect of HIV serostatus in our regression models was equivalent to approximately 6 years of aging. In the general population, the decline in gonadal function with aging has been associated with multiple adverse health outcomes, including cardiovascular diseases, DM, osteoporosis, bone fractures, and metabolic syndrome,1,2 although it is unclear if these relationships are causal.21,23-25 Ongoing clinical trials of testosterone replacement in older men will help answer this important question.40
Our study has several limitations. First, the age range of studied men was narrow and the follow-up time relatively short. Second, we did not correlate FT changes with clinical outcomes in this analysis, an area of emphasis for future MACS work. Third, the majority of our population was white so may not be generalizable to other racial groups. Fourth, specific ART regimens have been associated with differential changes in FT6 and use of testosterone replacement.41 The relationship between ART and FT is an important topic but beyond the scope of this analysis. Finally, only 50% of our population had biomarkers of inflammation/immune activation biomarkers available, and although differences by HIV infection observed in the larger cohort16 were also observed here. The study may have been underpowered to observe smaller effect sizes.
In conclusion, although HIV-infected and HIV-uninfected men experienced similar declines in FT over the 10-year study interval, FT was lower and had smaller diurnal variation among the HIV-infected men. Taken together, our findings may suggest a premature aging of the HPG axis in HIV-infected men based on blunting of the normal diurnal variation, but further studies are needed to test this hypothesis.
|
|
| |
| |
|
|
|