 |
 |
 |
| |
HPTN 067/ADAPT
Background and Methods and Cape Town Results:
|
| |
| |
Reported by Jules Levin
IAS 2015 Vancouver July 19-23
Linda-Gail Bekker; James Hughes; Rivet Amico; Surita Roux; Craig Hendrix; Peter L. Anderson; Bonnie J. Dye; Vanessa Elharrar; Michael J. Stirratt; Robert M. Grant
WEBCAST: https://www.youtube.com/watch?v=yDP6Ue2xA0c
CROI authors and affiliations: Linda-Gail Bekker1; James Hughes2; Rivet Amico4; Surita Roux3; Craig Hendrix5; Peter L. Anderson6; Bonnie J. Dye7; Vanessa Elharrar8; Michael J. Stirratt9; Robert M. Grant10
1Dept of Medicine and Institute of Infectious Disease and Molecular Medicine, Cape Town, South Africa; 2HIV Prevention Trials Network, Seattle, WA, US; 3The Desmond Tutu HIV Centre, Cape Town, South Africa; 4University of Michigan, Ann Arbor, MI, US; 5Johns Hopkins University, Baltimore, MD, US; 6University of Colorado, Aurora, CO, US; 7FHI 360, Durham, NC, US; 8PSP/DAIDS/NIAID/NIH, Bethesda, MD, US; 9Center for Mental Health Research on AIDS, Bethesda, MD, US; 10University of California, San Francisco, CA, US
Showing our study in context of released of results from other PrEP studies.
Cape Town enrollment began in September 2011, follow up ended in June 2013.
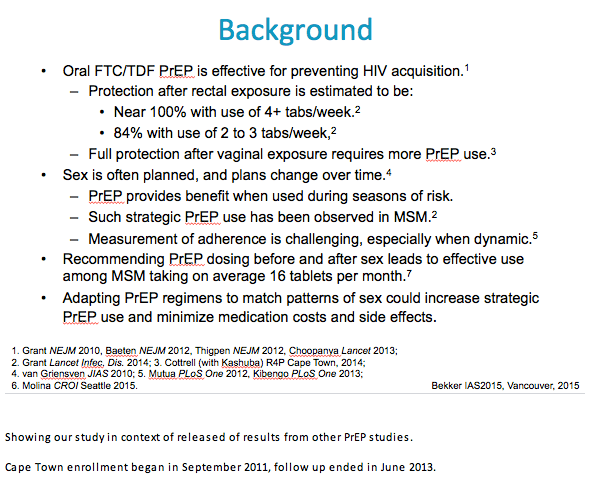
DOT was once a week to establish individual level pharmacology levels.
After 6 weeks of DOT to estimate steady state drug levels, participants were randomly assigned to one of three unblinded PrEP dosing regimens for 24 weeks of self-administered dosing as follows:
Daily (D)
Time Driven: Twice weekly with a post-intercourse boost (T)
Event-driven: Before and after intercourse (E)
Pills were dispensed from an electronic dispensing Wisepill device that recorded each opening
Participants were contacted weekly by phone or in person to review Wisepill data and sex events
Emphasize that collection of reported pill taking and sex events was not combined in the interview to reduce social desirability bias.
Final study visit at 34 weeks, 4 weeks after ending self-administered dosing
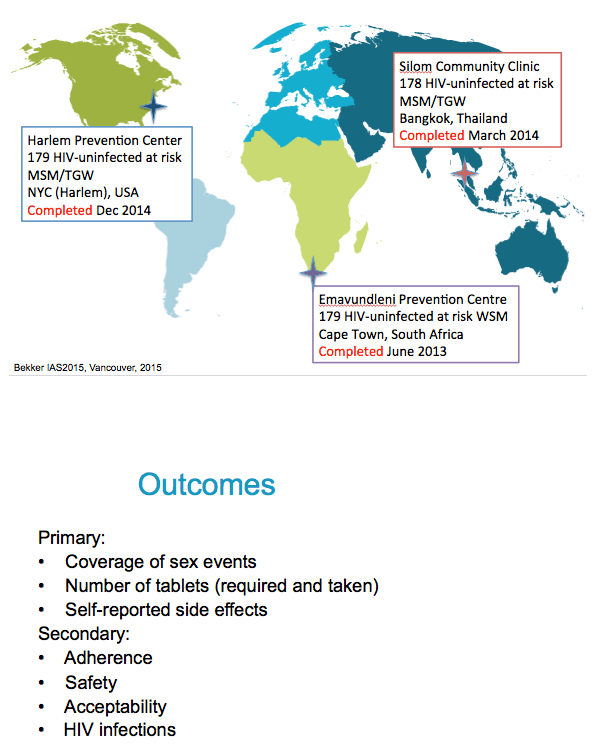
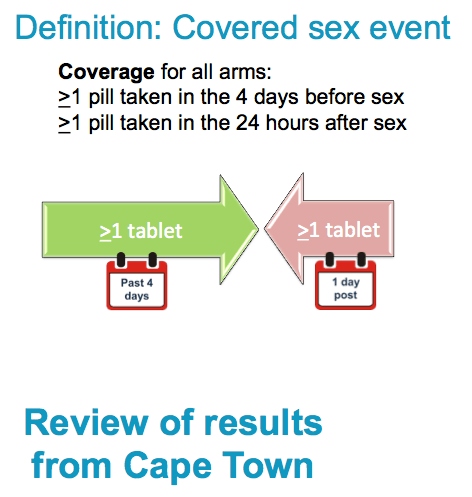
Coverage
Definition is the same for all 3 arms.
Only vaginal and anal sex acts will be considered; oral sex acts will not be included.
Periods of product hold (prior to acquisition of HIV) are included
The date and time of the sex act will be compared to the data and time of pill use as reported on item 2 of the Weekly Interview Log. A sex act will be considered "covered" if the following two conditions are met:
i) At least one pill is taken during the 96 hours before the act
ii) A pill is taken within 24 hours after the act
Note that the same pill can cover a post-exposure dose for one event and a pre-exposure dose for a different event.
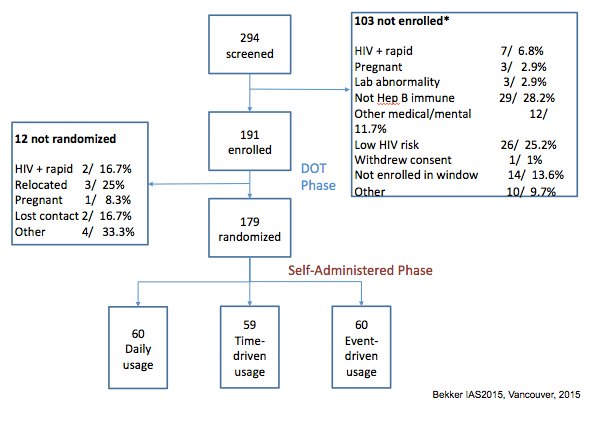
·More than one reason can be noted for reason not enrolled.
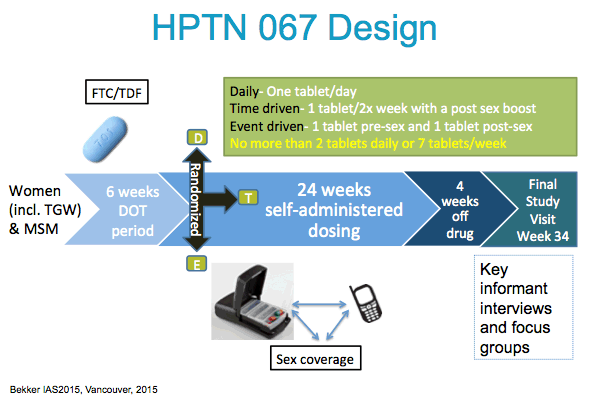
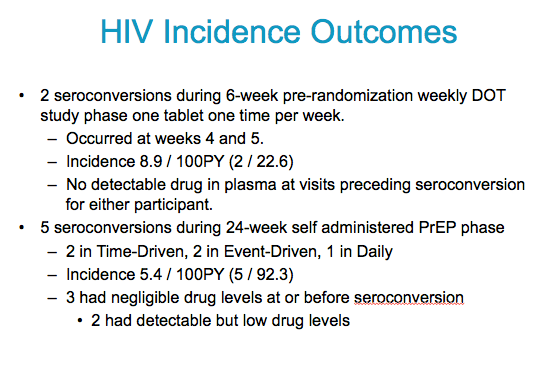
191 women enrolled in the DOT phase
179 randomized to the self administered phase
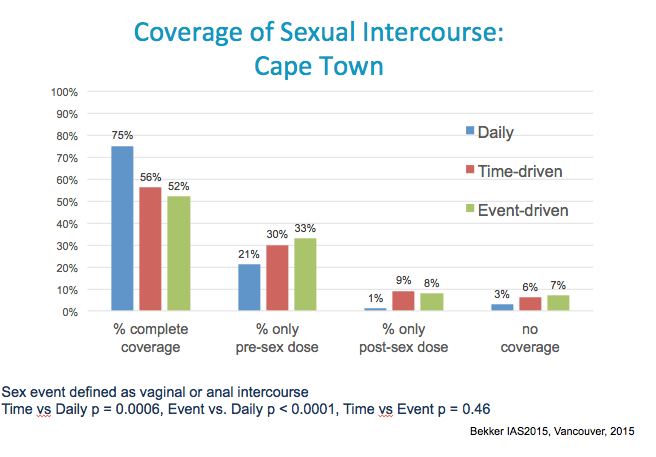
Coverage (yes vs. no) 0.42( 0.26, 0.69) 0.0006 0.36( 0.22, 0.59) <.0001 1.17( 0.78, 1.75) 0.4584 0.0005
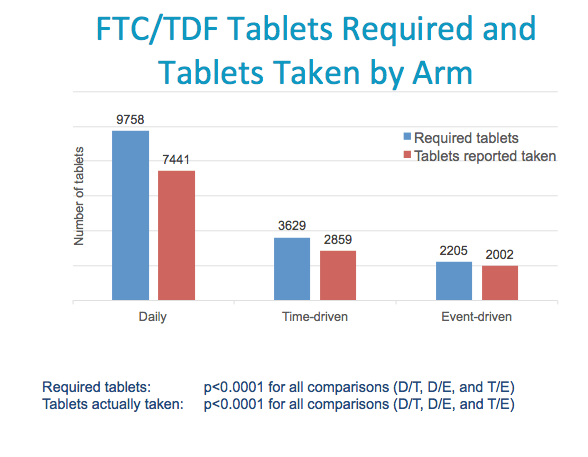
Another primary outcome was the number of tablets required and taken
The number of tablets required, shown in blue, was calculated by the number required by each dosing regimen over study follow-up (adjusted by the #of reported sex acts for time- & event-driven arms
The number of required tablets differed significantly by arms- daily required the most, event-driven required the fewest tablets
Est(95% CI) p-value Est(95% CI) p-value Est(95% CI) p-value global p-value
Time-drive vs. Daily Event-driven vs. Daily Time-driven vs. Event-driven
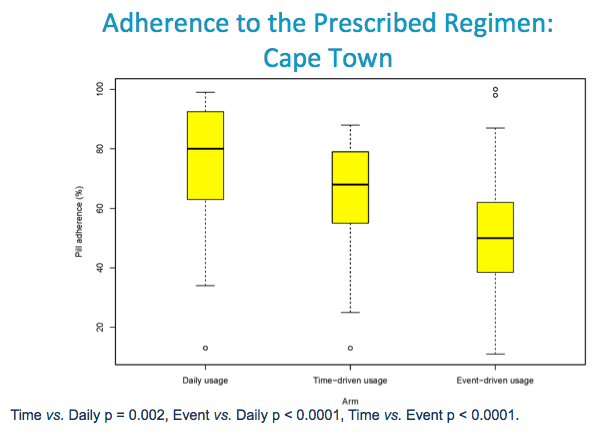
Adherence (Mean difference) -0.10(-0.17, -0.04) 0.0020 -0.24(-0.30, -0.18) <.0001 0.14( 0.07, 0.20) <.0001 <.0001
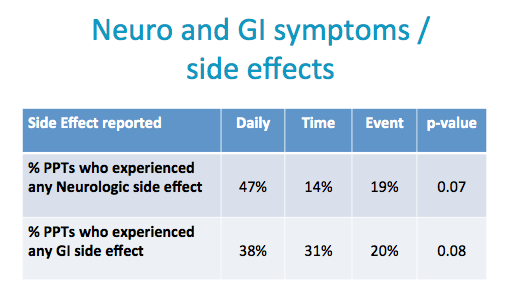
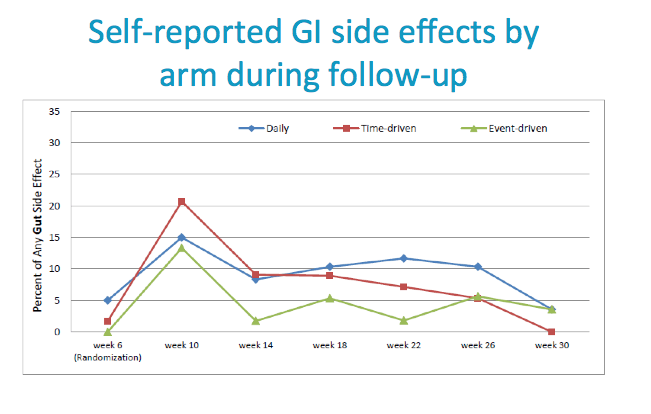
Neurologic side effects (such as headache, dizziness, lightheadedness) and Gastrointestinal side effects (such as nausea, vomiting, diarrhea, gas, bloating and abdominal pain) were commonly reported.
Evidence of a start up syndrome in all arms.
Neurologic side effects (such as headache, dizziness, lightheadedness)
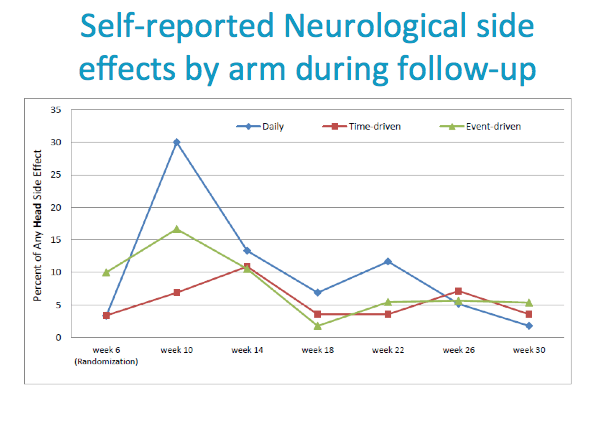
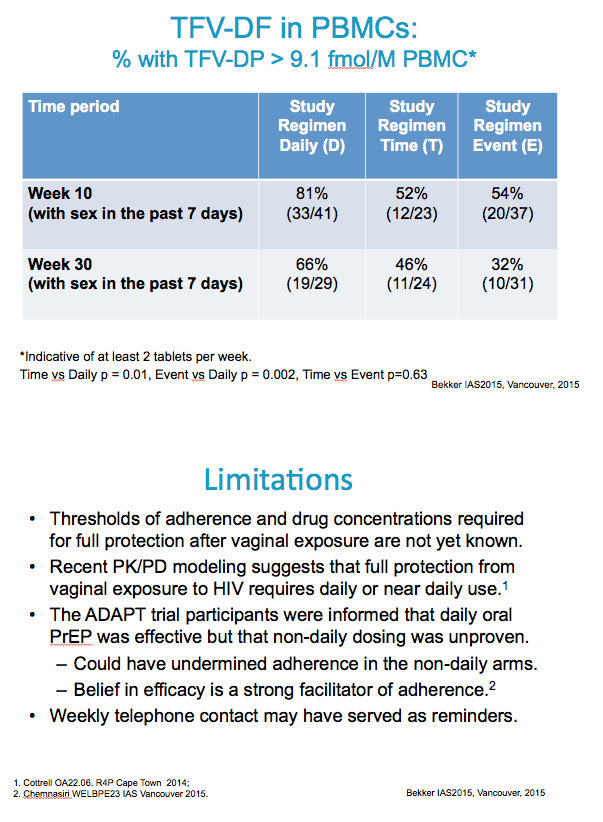
TFVDP in PBMC (yes vs. no) among people who reporting
having sex in the last 7 days
Time-drive vs. Daily Event-driven vs. Daily Time-driven vs. Event-driven
OR (95% CI) p-value OR (95% CI) p-value OR (95% CI) p-value global p-value
0.33( 0.14, 0.78) 0.0120 0.27( 0.12, 0.61) 0.0016 1.21( 0.54, 2.71) 0.6360 0.0028
|
| |
|
 |
 |
|
|