 |
 |
 |
| |
Safety and Efficacy of TAF vs TDF Single-Tablet Regimen in HIV-1 Treatment-Na´ve Black and Nonblack Patients Through Week 48
|
| |
| |
Reported by Jules Levin
IDSA 2015, October 7-11, 2015, San Diego, CA
David Wohl,1 Joseph Gathe,2 Melanie Thompson,3 Robert Grossberg,4 Sorana Segal-Maurer,5 Laurent Cotte,6 Indira Brar,7 Susan Guo,8 Devi SenGupta,8 Marshall Fordyce8
1The University of North Carolina at Chapel Hill, NC; 2Therapeutic Concepts, Houston, TX; 3AIDS Research Consortium of Atlanta, GA; 4Albert Einstein College of Medicine, Bronx, NY; 5New York-Presbyterian Queens, Flushing, NY; 6Hospices Civils de Lyon, France; 7Henry Ford Hospital, Detroit, MI; 8Gilead Sciences, Inc., Foster City, CA
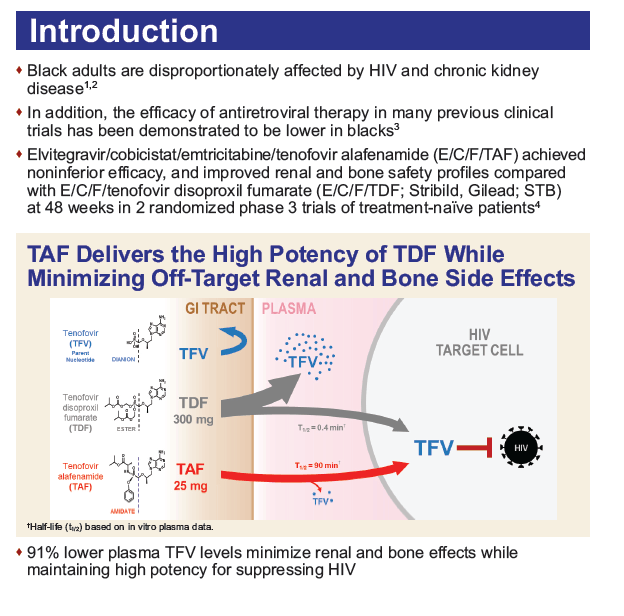
Background: Black adults are disproportionately affected by HIV and chronic kidney disease. We report 48 week efficacy and safety data of tenofovir alafenamide (TAF) vs. tenofovir disoproxil fumarate (TDF) in Black and non-Black subjects from two phase 3 treatment-na´ve trials.
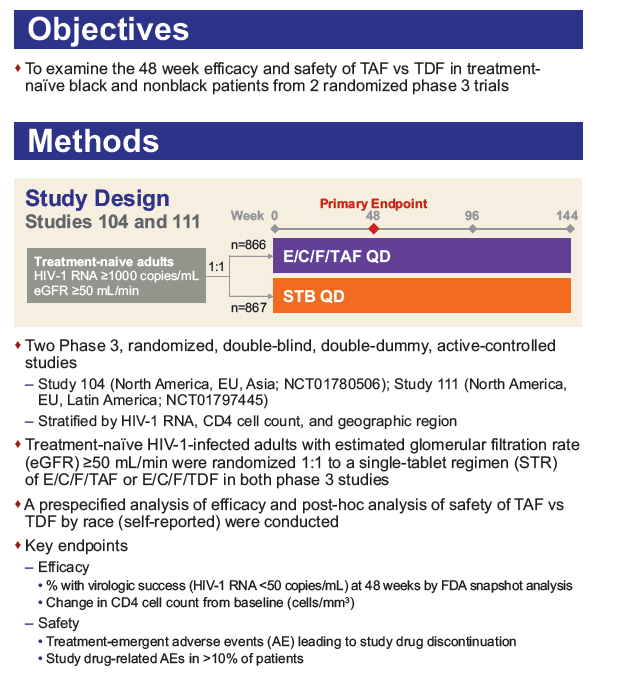
Methods: Treatment-na´ve HIV-1-infected adults with eGFR ≥50 mL/min were randomized 1:1 to a single tablet regimen (STR) of elvitegravir/cobicistat/emtricitabine/TAF (E/C/F/TAF) or elvitegravir/cobicistat/emtricitabine/TDF (E/C/F/TDF) in both studies. A pre-specified analysis of efficacy and a post-hoc analysis of safety of TAF vs. TDF by race were conducted.
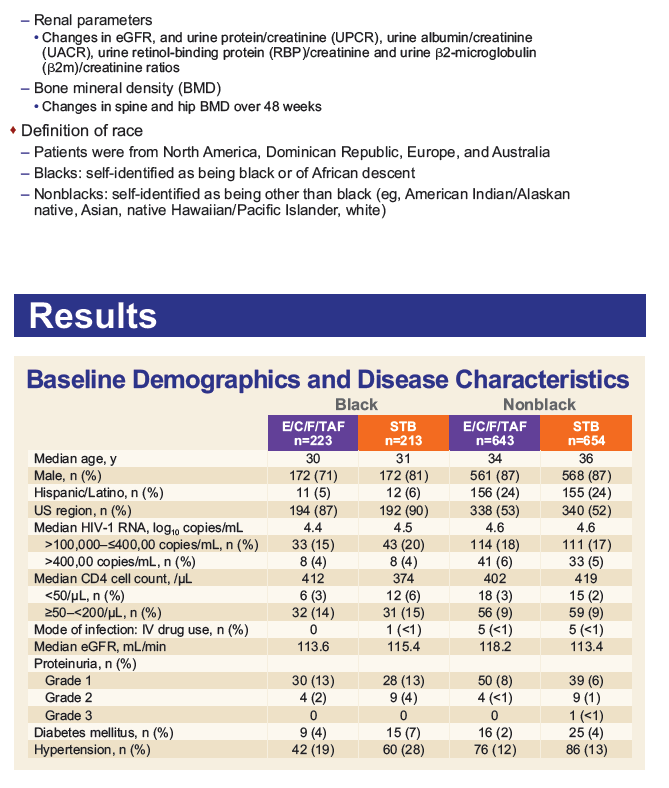
Results: Of the 1733 subjects treated, 436 (25%) self-identified as Black. Baseline characteristics among Blacks were balanced between treatment arms.
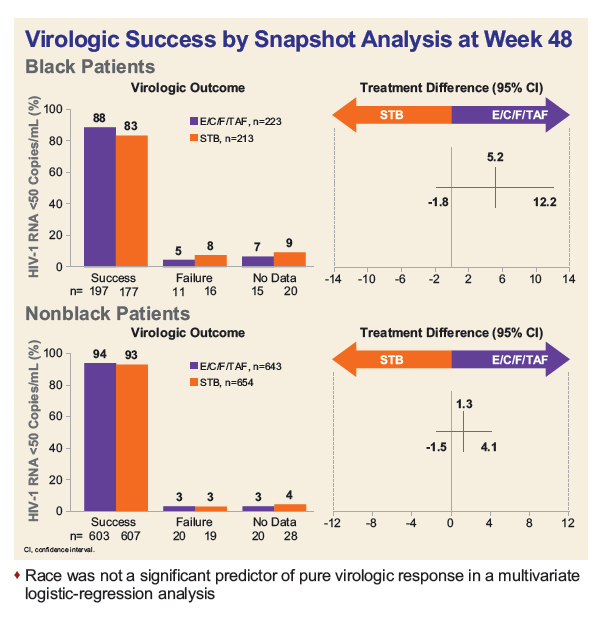
- At Week 48, rates of virologic success were numerically (though not statistically) higher for Blacks on TAF vs. TDF (88% vs. 83%), but significantly lower than in non-Blacks (94% TAF vs. 93% TDF).
- In the TAF arm, the overall lower rate of success in Blacks was primarily driven by lack of virologic data (6.7% Black vs. 3.1% Non-black, p=0.018), with most discontinuing study drug due to reasons other than adverse events.
- In the TDF arm, both virologic failure (p=0.003) and missing virologic data were higher in Blacks vs. non-Blacks (p=0.005).
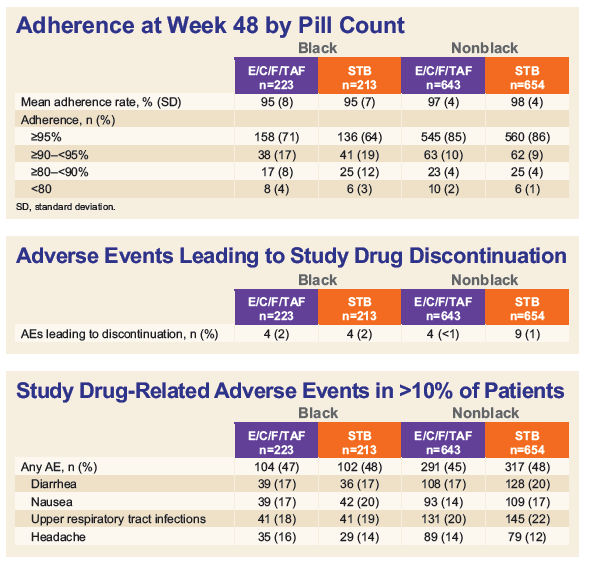
- Pill-count adherence was also lower in Blacks vs. non-Blacks in both the TAF (mean 95.4% vs. 97.3%; p<0.001) and TDF groups (95.1% vs. 97.6%; p<0.001).
- Both STRs were well-tolerated and proximal renal tubulopathy was not observed.
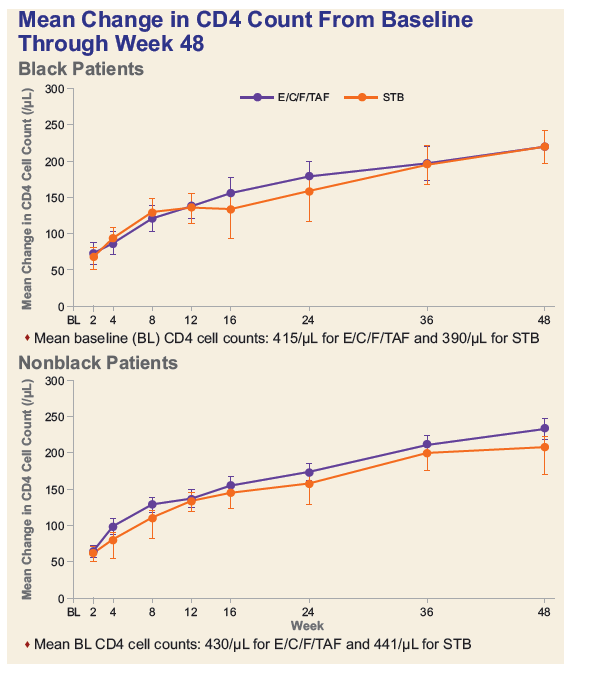
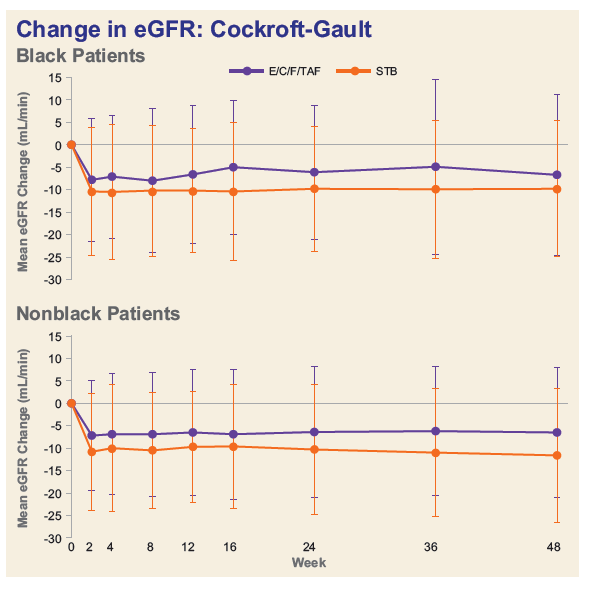
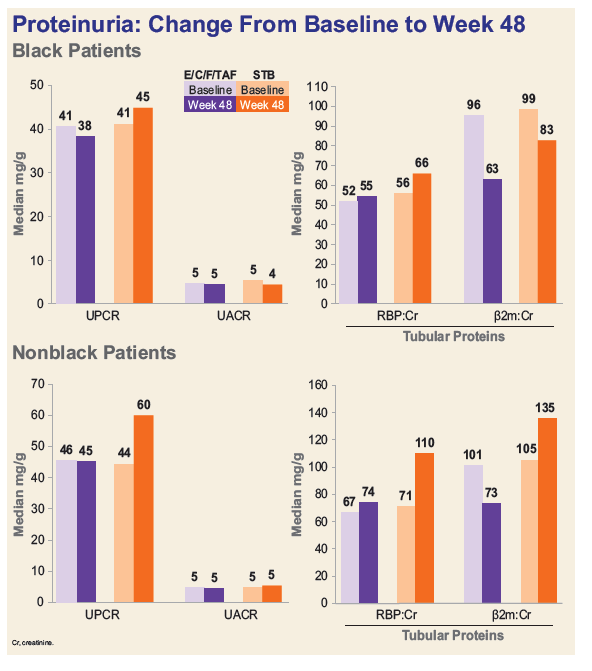
- Among Blacks, decline in median eGFR was significantly less and median decreases in proteinuria were numerically greater in the TAF group.
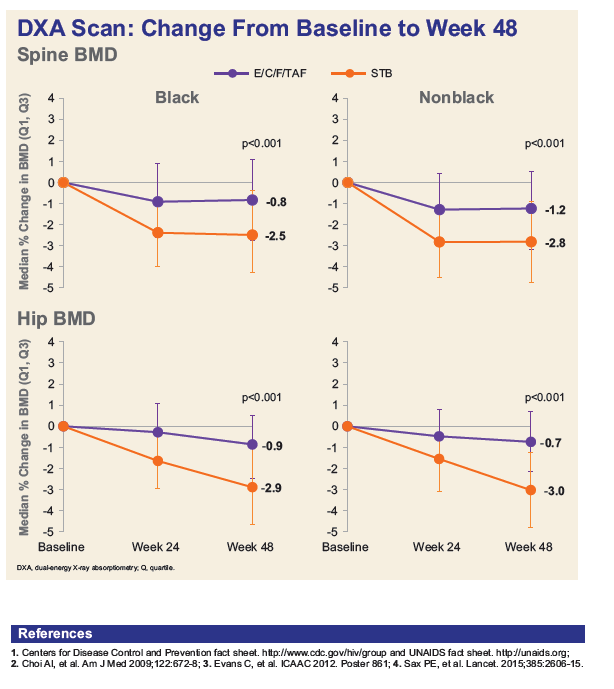
- Blacks experienced similar bone mineral density declines compared with non-Blacks, while those on TAF had less spine and hip BMD loss vs. TDF in both populations.
Conclusion: In treatment-na´ve Black adults, E/C/F/TAF demonstrates significant improvements in renal and bone safety over E/C/F/TDF with similar high-level efficacy at Week 48. Rates of virologic success and adherence were high for Blacks but were lower than those for non-Blacks. These results support the use of E/C/F/TAF for the initial treatment of HIV-1 in Black and non-Black adults.
|
| |
|
 |
 |
|
|