 |
 |
 |
| |
Triple-DAA Combo Cuts SSRI Levels by One Third, But No Dose Adjustment Needed
|
| |
| |
slides below....
16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy, May 26-28, 2015, Washington, DC
Mark Mascolini
Taking DCV-TRIO (daclatasvir, asunaprevir, and beclabuvir) lowered concentrations of two selective serotonin reuptake inhibitors (SSRIs), escitalopram (Lexapro) and sertraline (Zoloft), in healthy volunteers [1]. Bristol-Myers Squibb (BMS) researchers who conducted the study advised that dose adjustments of the SSRIs are not needed but that package-insert monitoring recommendations for the two SSRIs should be followed.
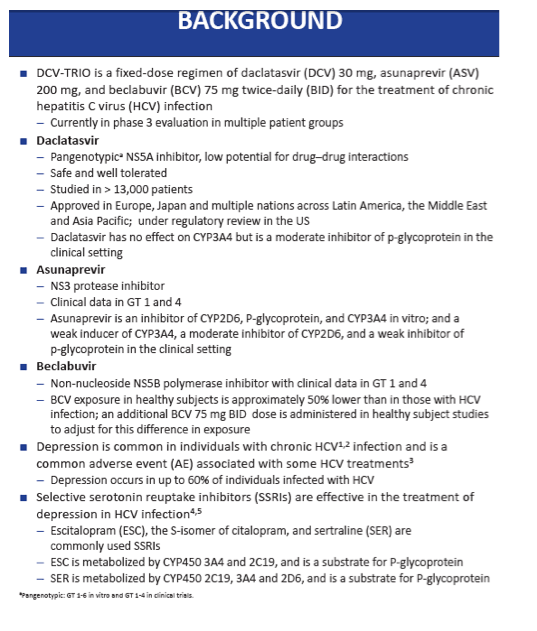
Because depression often affects people with HCV infection, those prescribed contemporary direct-acting antiviral (DAA) regimens may also be taking SSRIs like escitalopram and sertraline, which are metabolized by the enzymes CYP3A4 and CYP2C19 and are substrates for P-glycoprotein (P-gp). Asunaprevir weakly induces CYP3A4 and weakly inhibits P-gp in vivo, daclatasvir moderately inhibits P-gp in vivo, and DCV-TRIO moderately induces CYP2C19.
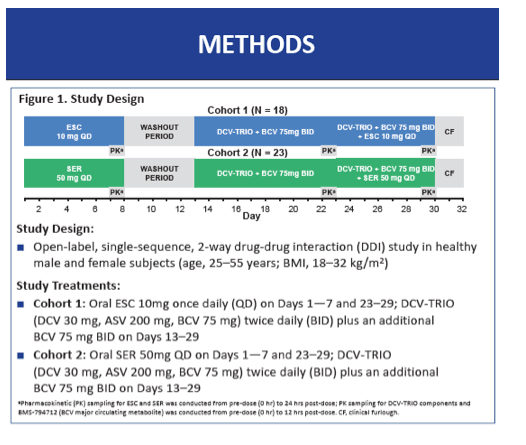
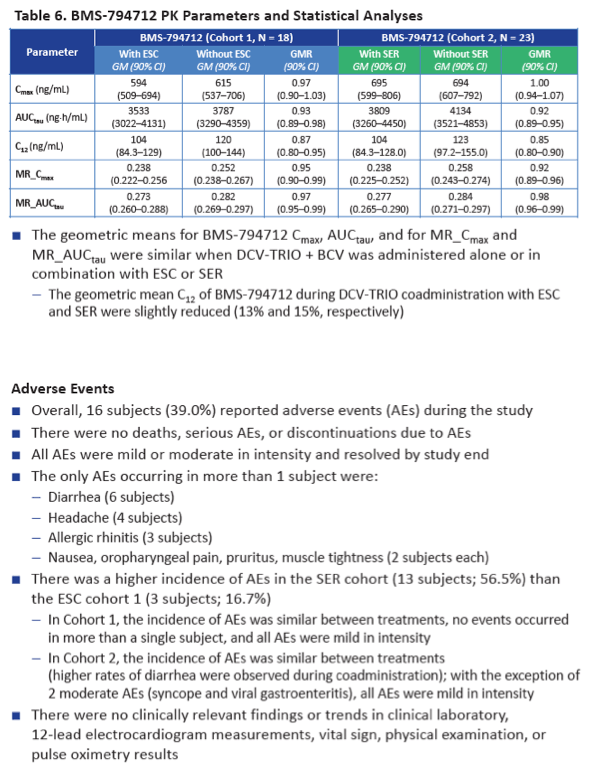
To determine whether components of DCV-TRIO affect pharmacokinetics of these two SSRIs, and vice versa, a BMS team conducted this two-cohort study in healthy volunteers 25 to 55 years old and with a body mass index between 18 and 32 kg/m(2). Cohort 1 took 10 mg of escitalopram once daily on days 1 through 7 and days 23 through 29, while cohort 2 took 50 mg of sertraline once daily on those days. The two dosing intervals were separated by a no-drug washout of several days. All participants took twice-daily DCV-TRIO on days 13 through 29; they also took an extra 75 mg of beclabuvir twice daily to adjust for exposure differences between people with and without HCV infection. The investigators calculated geometric mean ratios (GMR) and 90% confidence intervals (CI) for each drug plus the beclabuvir metabolite BMS-794712 for area under the concentration-time curve in one dosing interval (AUCtau), 24-hour concentration (C24), and maximum concentration (Cmax).
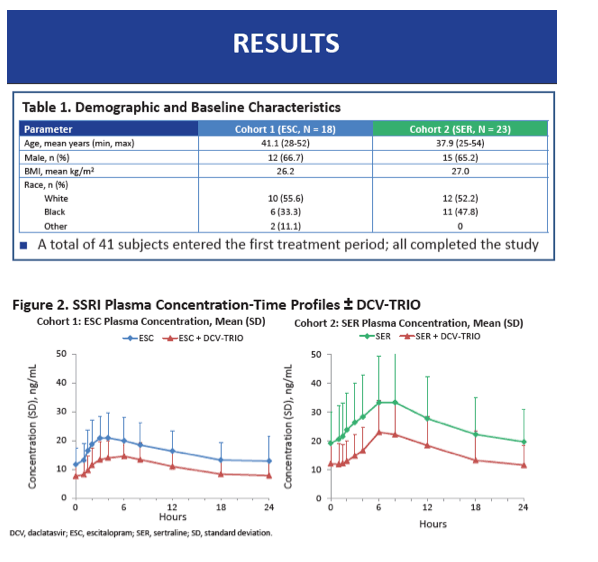
The 18 participants in Cohort 1 averaged 41.1 years in age, while the 23 in Cohort 2 averaged 37.9 years. Body mass index averaged 26.2 kg/m(2) in Cohort 1 and 27.0 kg/m(2) in Cohort 2. Two thirds of each cohort were men, and a little over half were white. All participants completed the study.
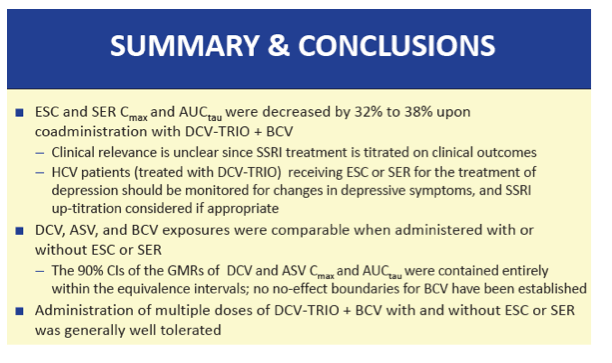
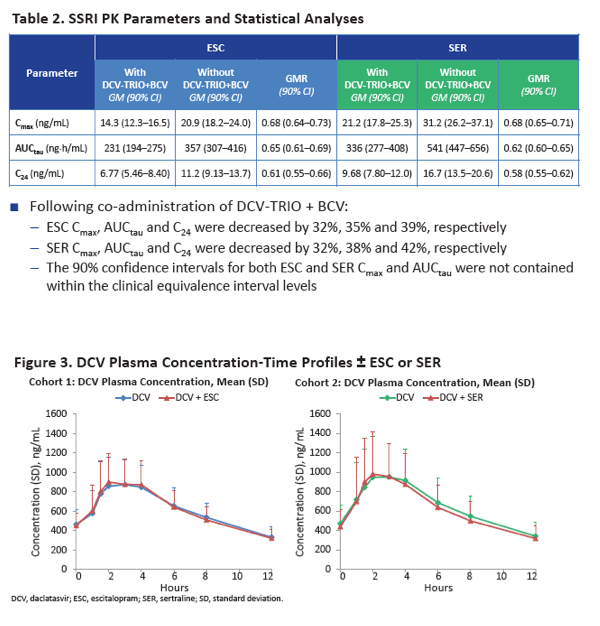
When taken with DCV-TRIO plus the extra 75 mg of beclabuvir twice daily, escitalopram AUCtau was 35% lower than without the DAAs (GMR 0.65, 90% CI 0.61 to 0.69) and sertraline AUCtau was 38% lower (GMR 0.62, 90% CI 0.60 to 0.65). Escitalopram C24 was 39% lower with than without DCV-TRIO (GMR 0.61, 90% CI 0.55 to 0.66), while sertraline C24 was 42% lower (GMR 0.58, 90% CI 0.55 to 0.62). Cmax of both escitalopram and sertraline was 32% lower with DCV-TRIO.
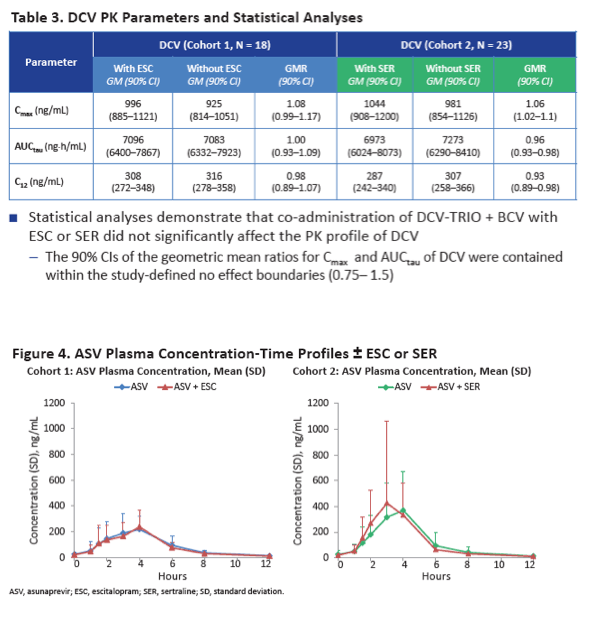
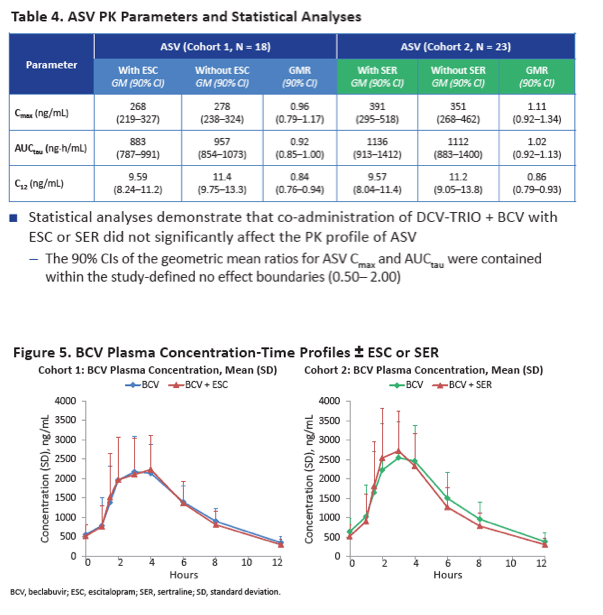
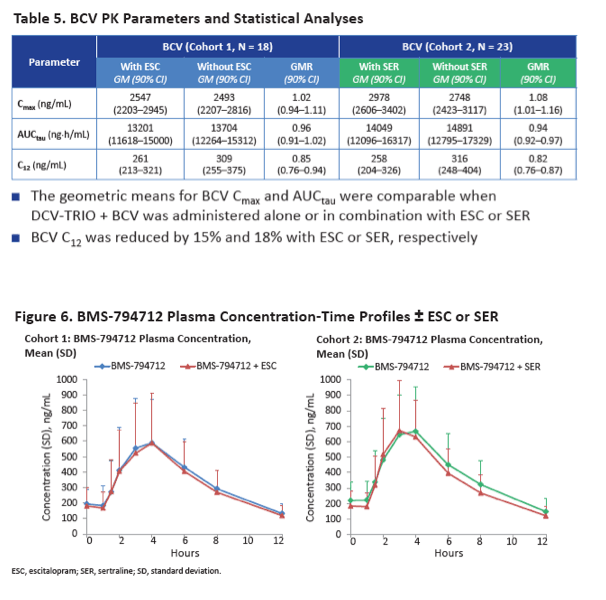
Neither escitalopram nor sertraline affected AUCtau or Cmax of daclatasvir, asunaprevir, beclabuvir, or BMS-794712.
There were no serious adverse events, discontinuations because of adverse events, or clinically relevant lab findings during the study. Adverse event incidence was higher with sertraline (13 people, 56.5%) than with escitalopram (3 people, 16.7%).
The researchers proposed that "no a priori escitalopram or sertraline dose adjustments are recommended during coadministration with DCV-TRIO." But people taking DCV-TRIO with escitalopram or sertraline "should be monitored for changes in depressive symptoms and SSRI up-titration considered if appropriate."
Reference
1. Tao X, Sims K, Hesney M, et al. The effect of daclatasvir, asunaprevir, and beclabuvir on the pharmacokinetics of selective serotonin reuptake inhibitors in healthy subjects. 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. May 26-28, 2015. Washington, DC. Abstract 81.
--------------------------
The Effect of Daclatasvir, Asunaprevir, and Beclabuvir on the Pharmacokinetics
of Selective Serotonin Reuptake Inhibitors In Healthy Subjects
Reported by Jules Levin
16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. Washington DC, USA, May 26-28, 2015
Tao X,1 Sims K,1 Hesney M,1 Stonier M, 1 Pursley J,1 Griffies A,1 Bhatnagar R,2 Smith C,2 Huang J,2 AbuTarif M1
1Bristol-Myers Squibb Research and Development, Princeton, NJ; 2PPD, Wilmington, NC


|
| |
|
 |
 |
|
|