 |
 |
 |
| |
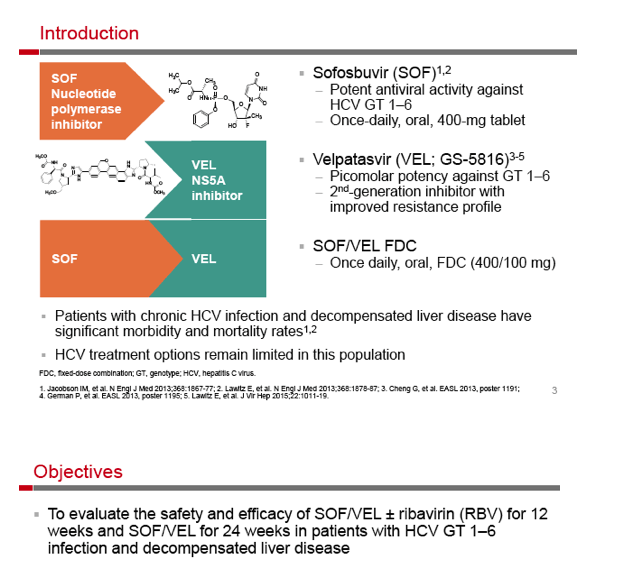
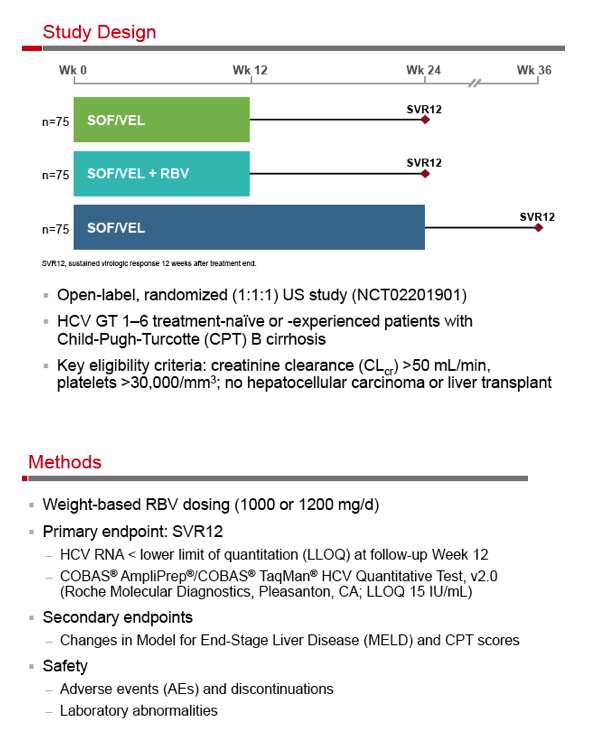
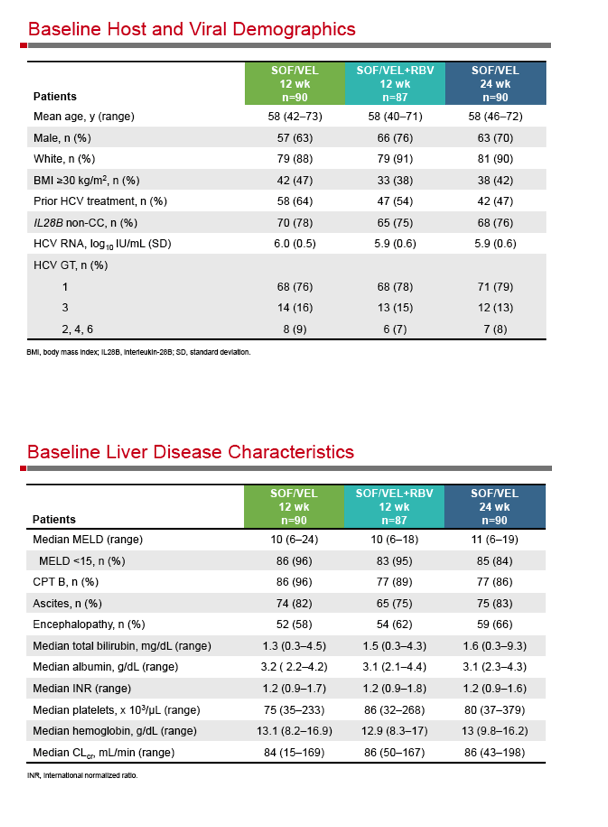
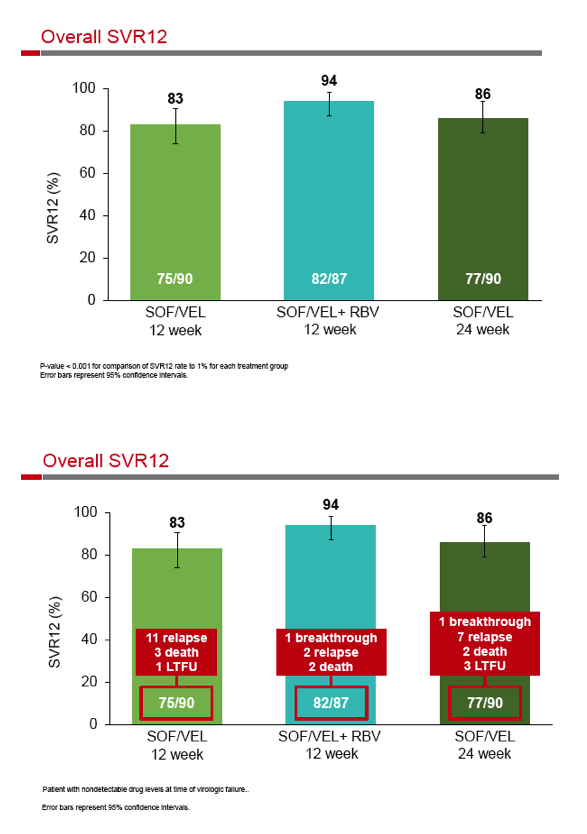
SOF/Velpatasvir, +GS-9857 - Sofosbuvir/Velpatasvir Fixed-Dose Combination for the Treatment of HCV in Patients With Decompensated Liver Disease: the Phase 3 ASTRAL-4 Study
|
| |
| |
"Gilead filed the NDA for SOF/VEL on October 28, 2015, and FDA has set a target action date under the Prescription Drug User Fee Act (PDUFA) of June 28, 2016. The FDA has assigned SOF/VEL a Breakthrough Therapy designation, which is granted to investigational medicines that may offer major advances in treatment over existing options. The NDA for SOF/VEL is supported by data from four Phase 3 ASTRAL trials, which evaluated the fixed-dose combination in hepatitis C genotypes 1-6. A marketing application for SOF/VEL is also under review in the European Union, and was validated by the European Medicines Agency (EMA) in December.".....http://www.natap.org/2016/HCV/010516_02.htm
Sofosbuvir/Velpatasvir Fixed-Dose Combination for the Treatment of HCV in Patients With Decompensated Liver Disease: the Phase 3 ASTRAL-4 Study
Reported by Jules Levin
APASL - 25th Conference of the Asian Pacific association for the Study of the Liver 2016
Tokyo, Japan
M.R. Charlton,1 J.G. O'Leary,2 N.H. Bzowej,3 A.J. Muir,4 K.M. Korenblat,5 J.M. Fenkel,6 K.R. Reddy,7 E. Lawitz,8 T.D. Schiano,9 L.W. Teperman,10 R.J. Fontana,11 E.R. Schiff,12 M.W. Fried,13 B. Doehle,14 D. An,14 J. McNally,14 A. Osinusi,14 M. Natha,14 D.M. Brainard,14 J.G. McHutchison,14 R.S. Brown,15 M.P. Curry16
1Intermountain Medical Center, Murray, UT; 2Baylor Research Institute, Dallas, TX; 3Ochsner Medical Center, New Orleans, LA; 4Duke University, Durham, NC; 5Washington University School of Medicine in Saint Louis, MO; 6Thomas Jefferson University, Philadelphia, PA; 7University of Pennsylvania School of Medicine, Philadelphia, PA; 8Texas Liver Institute, San Antonio, TX; 9Mount Sinai Hospital, New York, NY; 10NYU School of Medicine, New York, NY; 11University of Michigan, Ann Arbor, MI; 12University of Miami, Coral Gables, FL; 13University of North Carolina at Chapel Hill School of Medicine; 14Gilead Sciences, Inc., Foster City, CA; 15Columbia University Medical Center, New York-Presbyterian, New York, NY; 16Beth Israel Deaconess Medical Center, Boston, MA
CROI:Drug Interaction Studies Between Sofosbuvir/Velpatasvir and Boosted HIV ARV Regimens - (02/24/16)
AASLD: Sofosbuvir/Velpatasvir + GS-9857 for 6 or 8 Weeks in Genotype 1 or 3 HCV-Infected Patients - (11/16/15)
AASLD: Resistance Analysis of Treatment-Naïve and DAA-Experienced Genotype 1 Patients With and Without Cirrhosis Who Received Short-Duration Treatment With Sofosbuvir/Velpatasvir + GS-9857 - (12/18/15)
AASLD: Drug-Drug Interaction Studies Between Hepatitis C Virus Antivirals Sofosbuvir and Velpatasvir (GS-5816) and HIV Antiretoviral Therapies - (12/07/15)
AASLD: Characterization of HCV Resistance From a 3-Day Monotherapy Study of GS-9857, a Novel Pangenotypic NS3/4A Protease Inhibitor - (12/18/15)
hepDART: High Efficacy of Sofosbuvir/Velpatasvir In HCV Genotypes 1-6 Infected Patients With Cirrhosis: Pooled Data From the ASTRAL 1, 2 and 3 Trials (12/14/15)
hepDART: High Efficacy of Sofosbuvir/Velpatasvir Across 7 HCV Genotypes and 46 Subtypes: Pooled Data From the ASTRAL1, 2 and 3 Trials - (12/14/15)
Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection - (12/11/15)
AASLD: Sofosbuvir+GS-5816 GT 1-6: ASTRAL-1, ASTRAL-2, ASTRAL-3, ASTRAL-4 - (12/09/15)
AASLD: Sofosbuvir/Velpatasvir + GS-9857 for 6 or 8 Weeks in Genotype 1 or 3 HCV-Infected Patients - (11/16/15)
AASLD: A Phase 3 Double-Blind Placebo-Controlled Evaluation of Sofosbuvir/Velpatasvir Fixed-Dose Combination for 12 Weeks in Genotype 1, 2, 4, 5, 6 HCV-Infected Patients: Results of the ASTRAL-1 Study - (11/17/15)
AASLD: A Randomized Controlled Trial of Sofosbuvir/Velpatasvir Fixed-Dose Combination for 12 Weeks Compared to Sofosbuvir with Ribavirin for 12 Weeks in Genotype 2 HCV-Infected Patients: The Phase 3 ASTRAL-2 Study - (11/30/15)
AASLD: Sofosbuvir/Velpatasvir Fixed-Dose Combination for 12 Weeks Compared to Sofosbuvir with Ribavirin for 24 Weeks in Genotype 3 HCV-Infected Patients: The Randomized Controlled Phase 3 ASTRAL-3 Study - (11/30/15)
AASLD: Sofosbuvir/Velpatasvir Fixed-Dose Combination for the Treatment of HCV in Patients With Decompensated Liver Disease: the Phase 3 ASTRAL-4 Study / ASTRAL 1, 2 and 3 - (11/30/15)
AASLD: Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection - (11/30/15) published
AASLD: Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection - (11/30/15) published
AASLD: Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis (ASTRAL-4) - (11/30/15) published
AASLD: Sofosbuvir Plus Velpatasvir (GS-5816) Combination Therapy for Treatment-Experienced Patients With Genotype 1 or 3 Hepatitis C Virus Infection: A Randomized Trial - (11/17/15) published

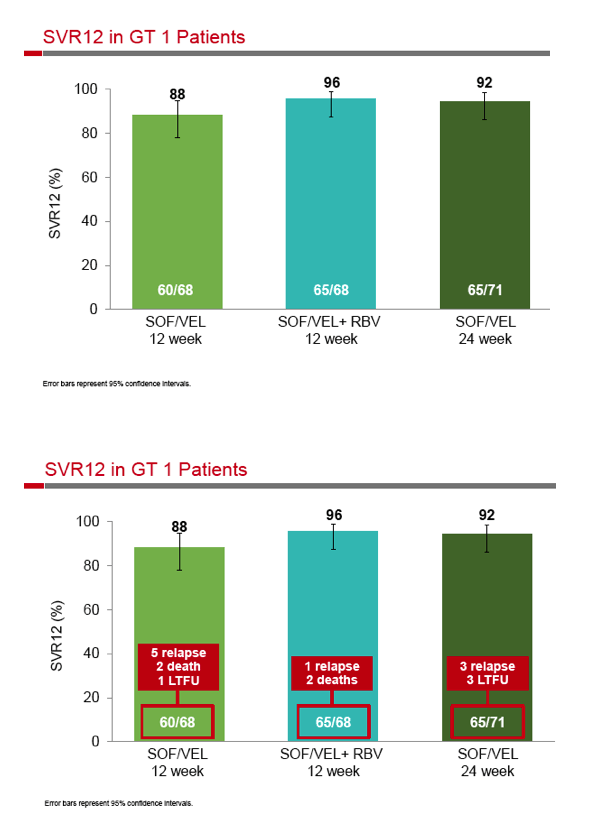
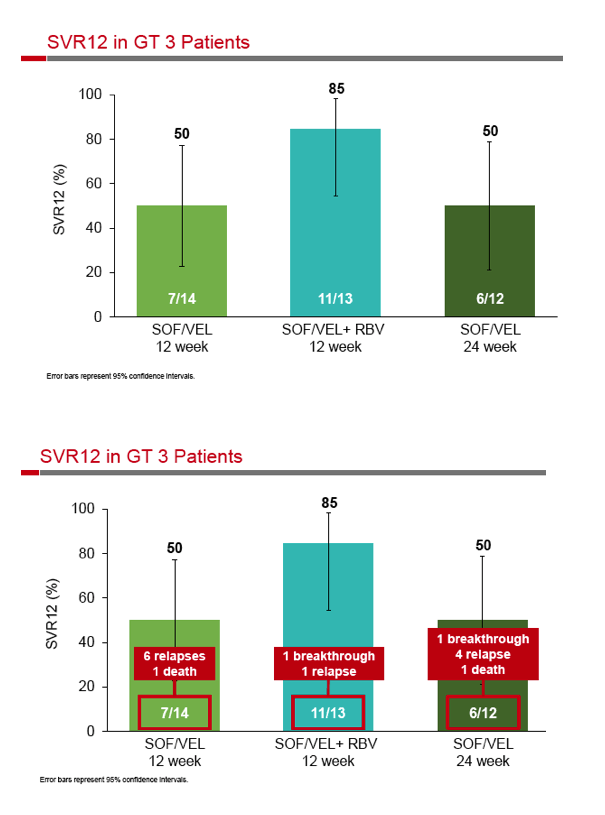
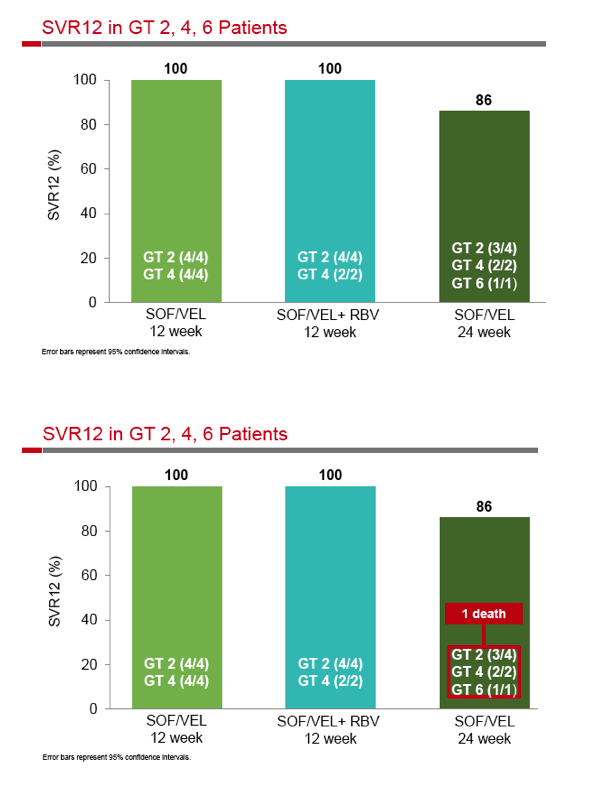
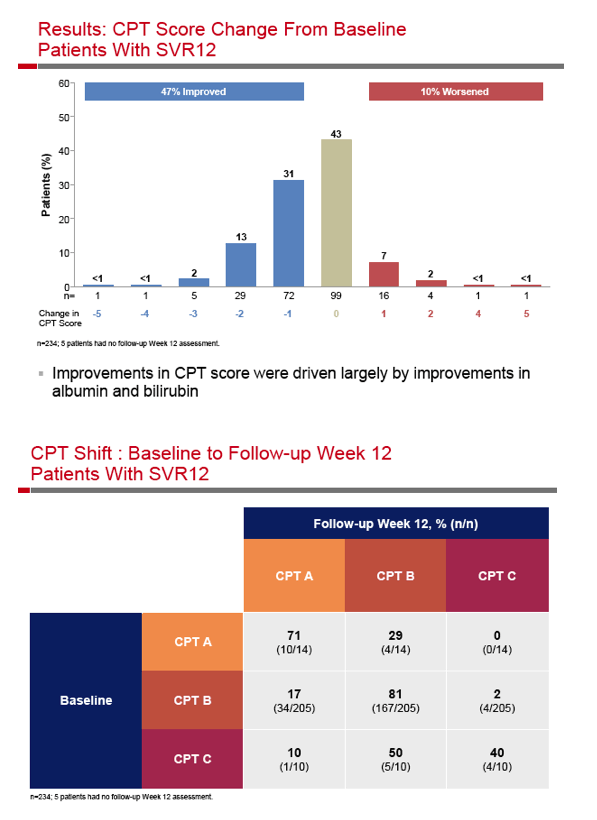
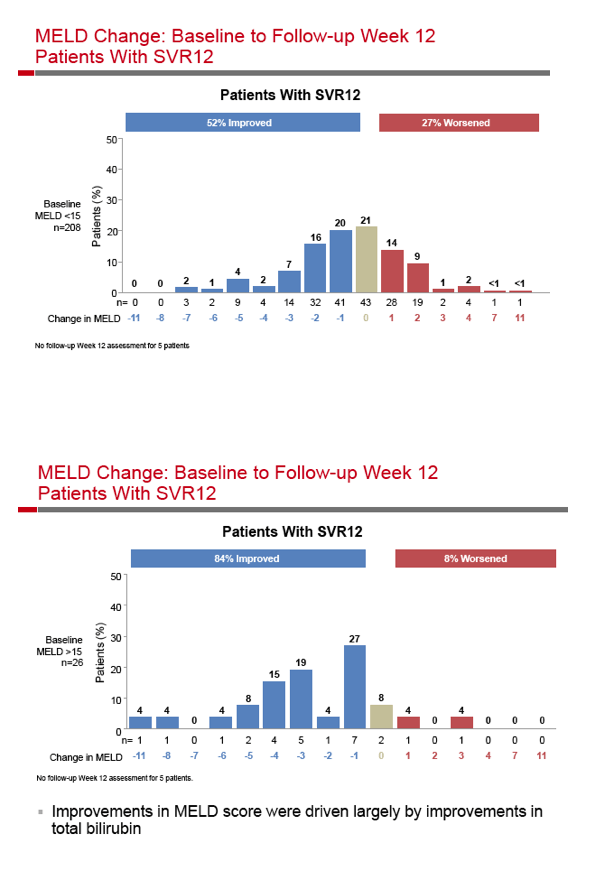
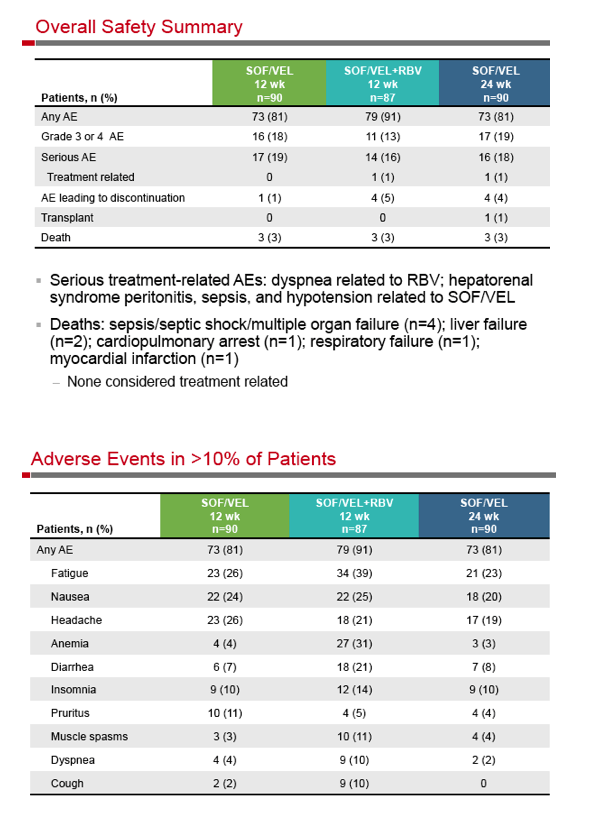
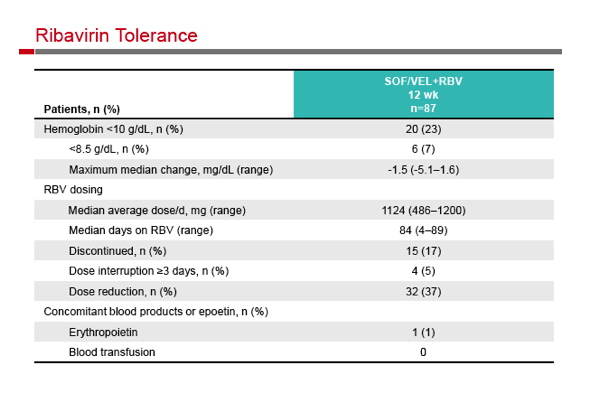
|
| |
|
 |
 |
|
|