 |
 |
 |
| |
Cabotegravir + Rilpivirine as Long-Acting Maintenance
Therapy: LATTE-2 Week 32 Results
|
| |
| |
Reported by Jules Levin
CROI 2016 Feb 22-24 Boston
David A. Margolis1; Juan Gonzalez-Garcia2; Hans-Jurgen Stellbrink3; Joseph J. Eron4; Yazdan Yazdanpanah5; Sandy Griffith6; David Dorey7; Kimberley Y. Smith1; Peter Williams8; William Spreen1
1ViiV Hlthcare, Research Triangle Park, NC, USA; 2Inst for Hlth Rsr of La Paz Univ Hosp, Madrid, Spain; 3ICH Study Cntr, Hamburg, Germany; 4Univ of North Carolina at Chapel Hill, Chapel Hill, NC, USA; 5INSERM U1137, Paris, France; 6ViiV Hlthcare, Durham, NC, USA; 7GSK, Mississauga, ON, Canada; 8Janssen, Beerse, Belgium
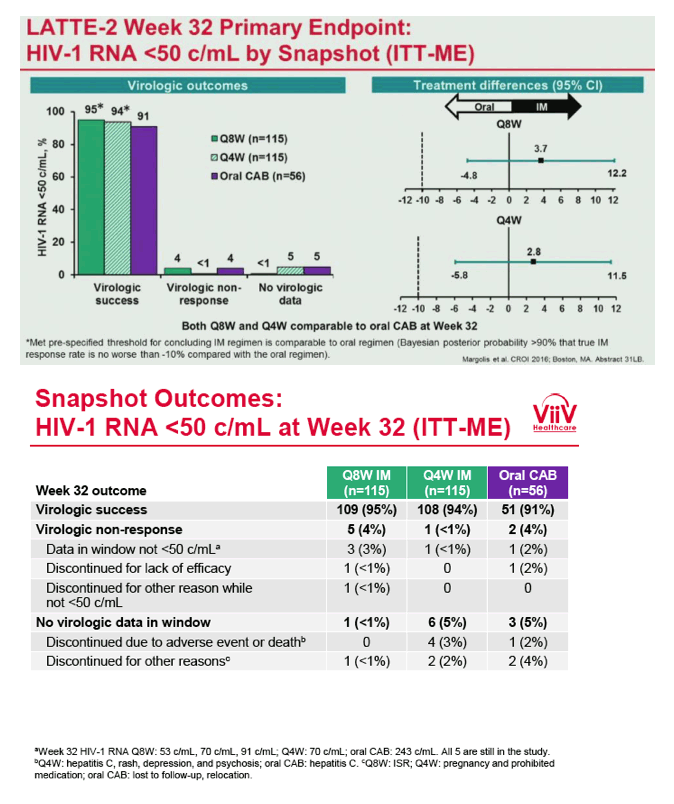
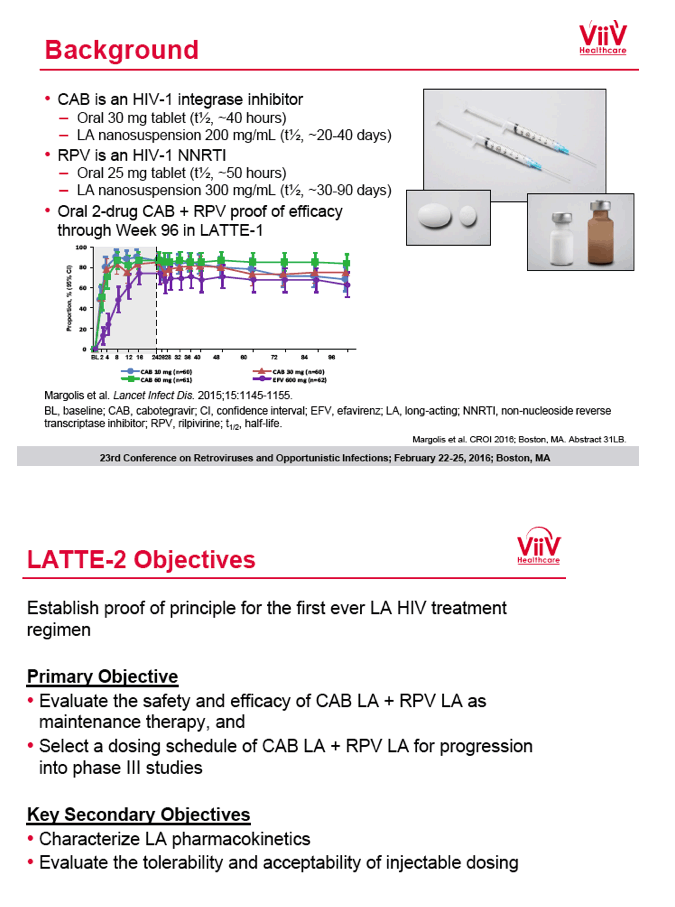
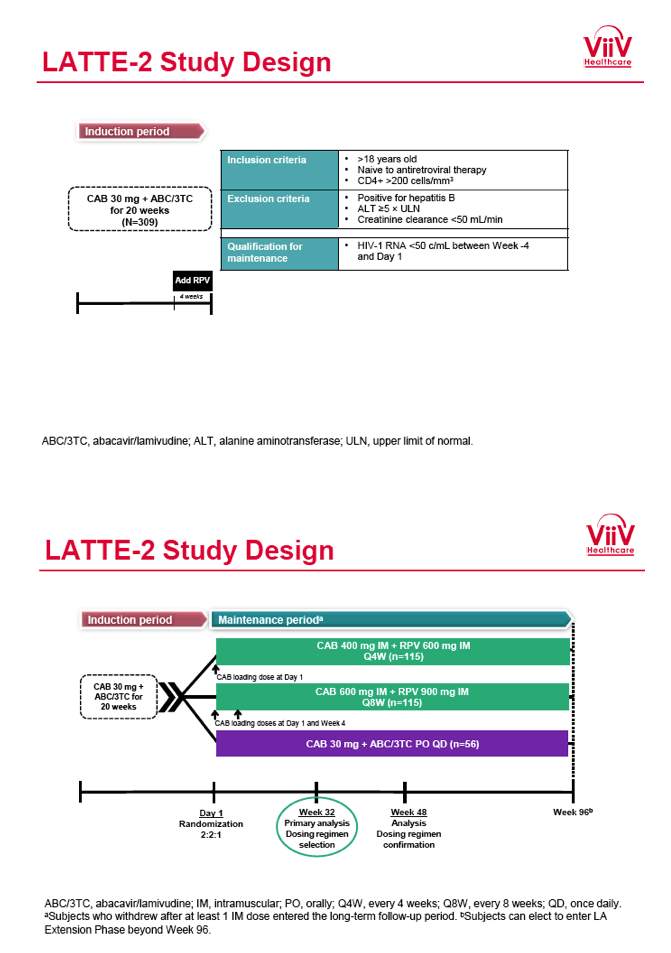
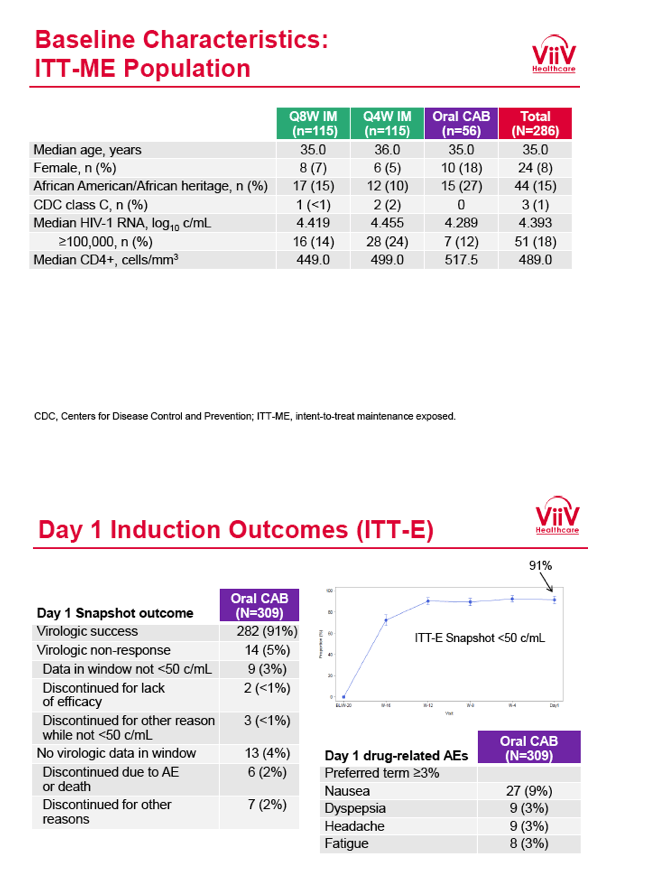
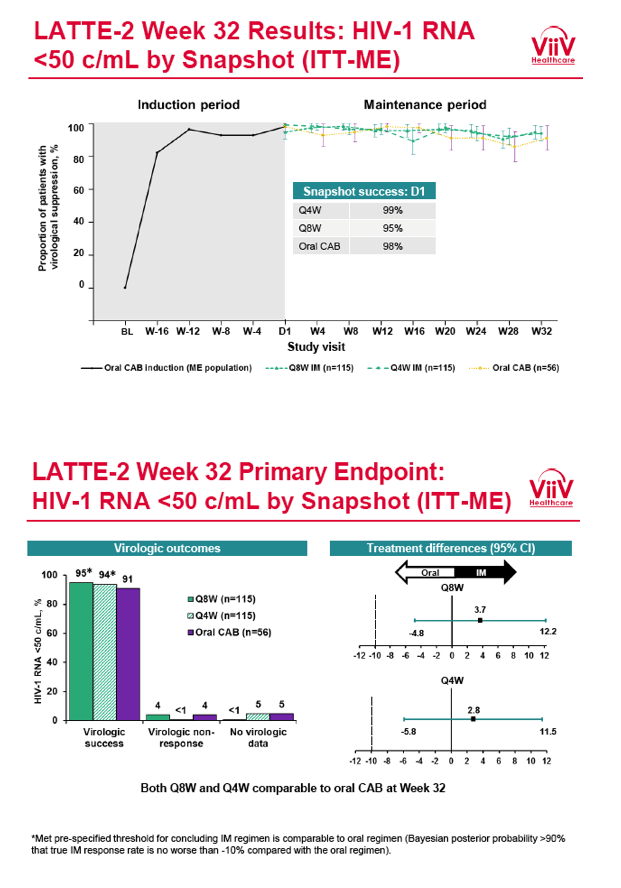
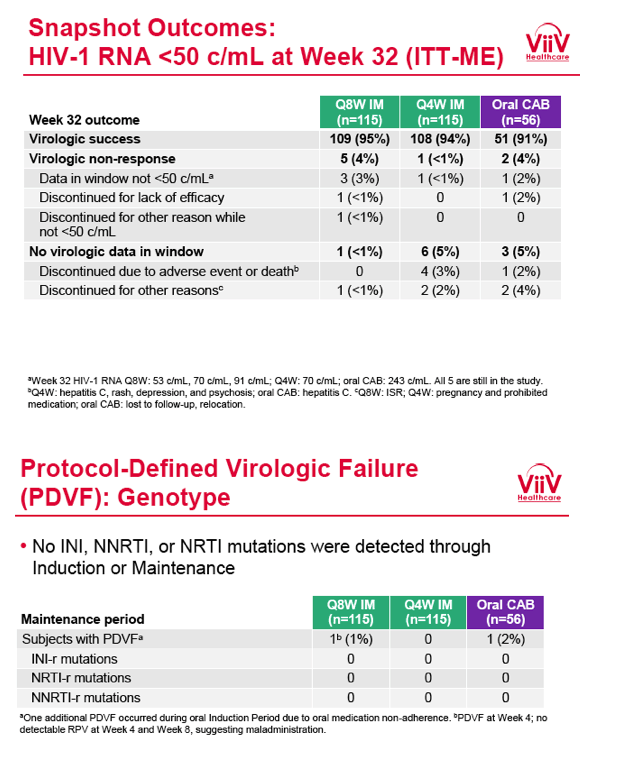
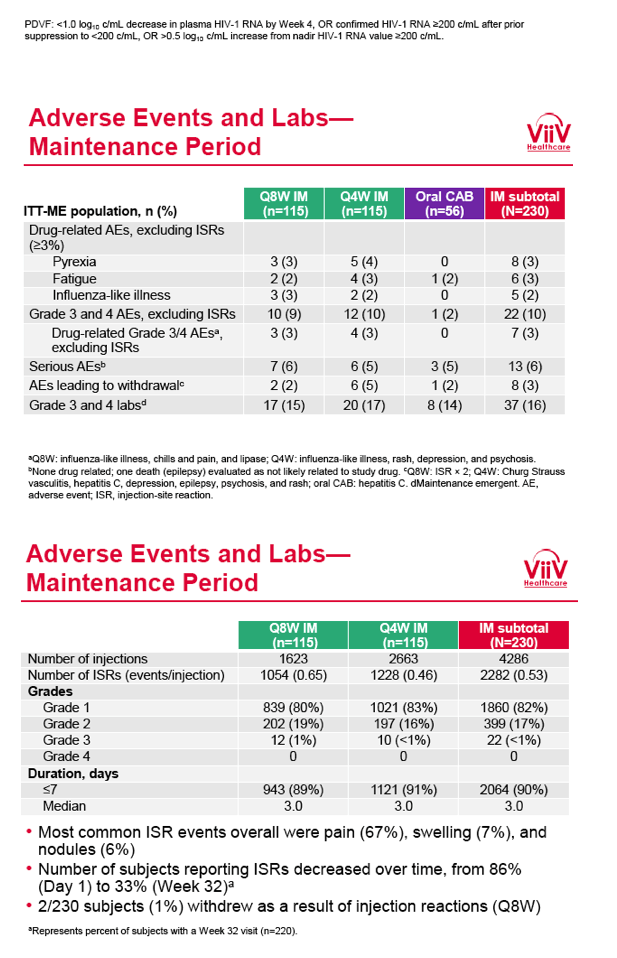
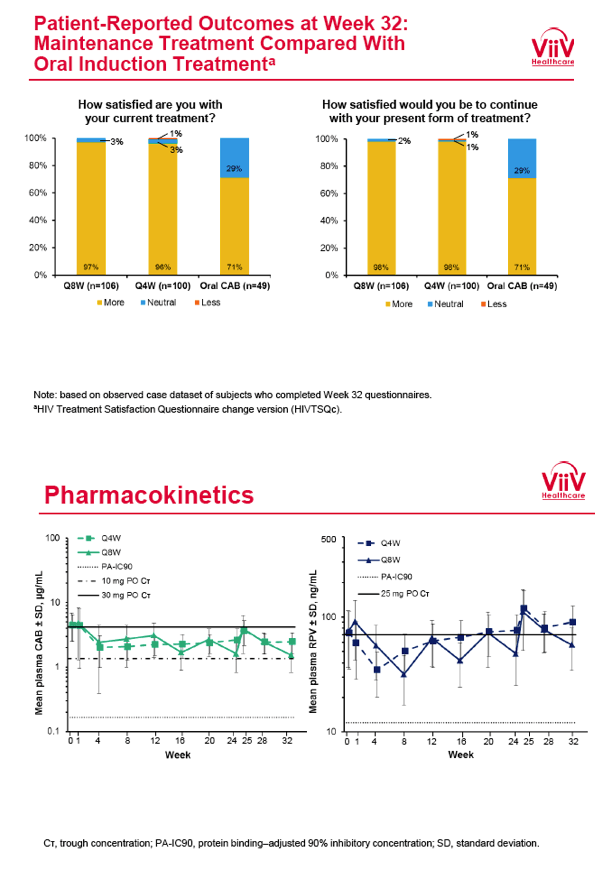
from Jules: purpose of LATTE-2 was to Establish proof of principle for the first ever LA [long-acting] HIV treatment regimen, evaluate the safety and efficacy of CAB LA + RPV LA as maintenance therapy, and select a dosing schedule of CAB LA + RPV LA for progression into phase III studies they also looked at long-acting pharmacokinetics for the regimen and safety and tolerability for injection dosing, initial efficacy for the oral form of CAB+RPV was established in LATTE-1 through 96 weeks. This was an induction/maintenance study - meaning initially 309 patients received for 20 weeks the oral form (pills) of Cabotegravir+abacavir/FTC once daily in order to achieve virology suppression, then for the final 4 weeks of the induction period rilpivirine 25 mg once daily was added. Patients with undetectable, <50 copies/ml viral load were permitted to enter the maintenance phase of this study where they were randomized to 1 of 3 study treatment arms. Patients could receive intramuscular injections every 4 weeks or 8 weeks or continue taking the oral form. At the end of the induction phase 95% of these treatment-naive patients had undetectable viral load. The primary endpoint of the study was the percent of patients with undetectable viral load at week 32: 95% on every 8 week injection dosing and 94% on every 4 weeks IM injection dosing had <50 c/ml, undetectable viral load, while 91% who continued on the oral dosing had undetectable viral load.
WEBCAST: http://www.croiwebcasts.org/console/player/29459?mediaType=audio&
|
| |
|
 |
 |
|
|