 |
 |
 |
| |
Comparable Viral Decay in Dual and Triple Dolutegravir-Based Antiretroviral Therapy
|
| |
| |
Omar G. Sued1; Maria I. Figueroa1; Maria J. Rolon1; Patricia Patterson2; Dannae Brown3; Ana M. Gun4; Michael Aboud5; Kimberley Y. Smith6; Pedro Cahn1
1Fundacion Huesped, Buenos Aires, Argentina;2Hosp Juan A. Fernandez, Buenos Aires, Argentina;3ViiV Hlthcare, Abbotsford, Australia;4Centro Medico Huesped, Buenos Aires, Argentina;5ViiV Hlthcare, Brentford, UK;6ViiV Hlthcare, Research Triangle Park, NC, USA
Reported by Jules Levin
CROI 2016 Feb 22-24 Boston
Program abstract
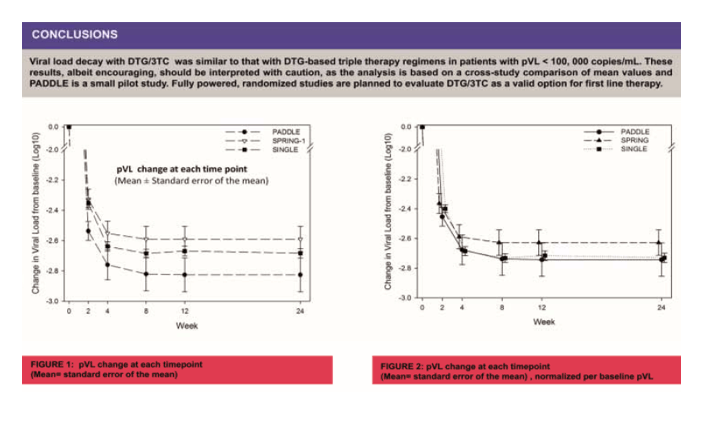
Drug-sparing strategies have been explored lately, aiming to improve tolerability and adherence, reduce toxicity, and costs. Two studies have shown non inferiority of dual therapy in ARV -naïve HIV-1 infected individuals (NEAT and GARDEL). The PADDLE study showed that a dual therapy regimen based on dolutegravir plus lamivudine (DTG/3TC) induced a rapid viral decay in treatment naïve patients with screening pVL <100K (EACS 2015). Our objective is to compare differences between plasma viral load (pVL) change at each time point with a dual therapy, DTG/3TC, to triple therapy regimens used in the SPRING-1 (DTG 50 mg +2NRTIs) and SINGLE study (DTG plus abacavir/lamivudine), in patients with baseline (BL) pVL < 100,000 copies/mL.
In PADDLE (n=20), pVL was tested at BL, days 2, 4, 7, 10, weeks 2, 4, 8, 12, 24 and thereafter. In SINGLE (n=280) it was measured at BL, wk 2, 4, 8, 12, 16, 24 and thereafter. In SPRING-1 (n=39) pVL was measured at BL, wk 1, 2, 4, 8, 12, 16, 20, 24 and thereafter. Change in pVL vs. baseline was calculated only for time points with data for the three studies (Weeks 2, 4, 8, 12 and 24). Effects of time and treatment, was analyzed by two-way ANOVA, followed by Tukey-HSD post-hoc test.
BL pVL (Mean+SD) was 4.43 (0.50), 4.30 (0.45) and 4.31 (0.52) for PADDLE, SINGLE and SPRING-1 respectively. Rapid decline in viral load was observed in the three regimens. Two-way ANOVA revealed significant effects for treatment (F2,1605=30.3 p<0.001) and time (F4,1605=22.8 p<0.001), without significant interactions. Average effects of treatment in PADDLE, SPRING-1 and SINGLE were -2.75±0.45 (Mean ±SD), -2.53±0.49 and -2.61±0.48 log10 respectively. Significant differences were observed between PADDLE and SPRING-1 or SINGLE studies (p<0.01 and p<0.05).
The figure shows the viral load change at each time point in the studies (Mean ± Standard error of the mean)
Viral load change was of similar magnitude after a dual therapy regimen DTG/3TC compared to 2 DTG-based triple therapy regimens. These results, albeit encouraging, should be interpreted with caution, as the analysis is based on a cross-study comparison of mean values and PADDLE is a small pilot study. Full powered, randomized studies are in progress to evaluate DTG/3TC as a valid option for first line therapy.

|
| |
|
 |
 |
|
|