 |
 |
 |
| |
ECLAIR: Phase 2A Safety and PK Study of Cabotegravir LA in HIV-Uninfected Men.......Long-Acting Cabotegravir Data Suggest PrEP Injections Every 8 Week
|
| |
| |
Conference on Retroviruses and Opportunistic Infections (CROI), February 22-25, 2016, Boston
Mark Mascolini
WEBCAST link: http://www.croiwebcasts.org/console/player/29581?mediaType=audio&
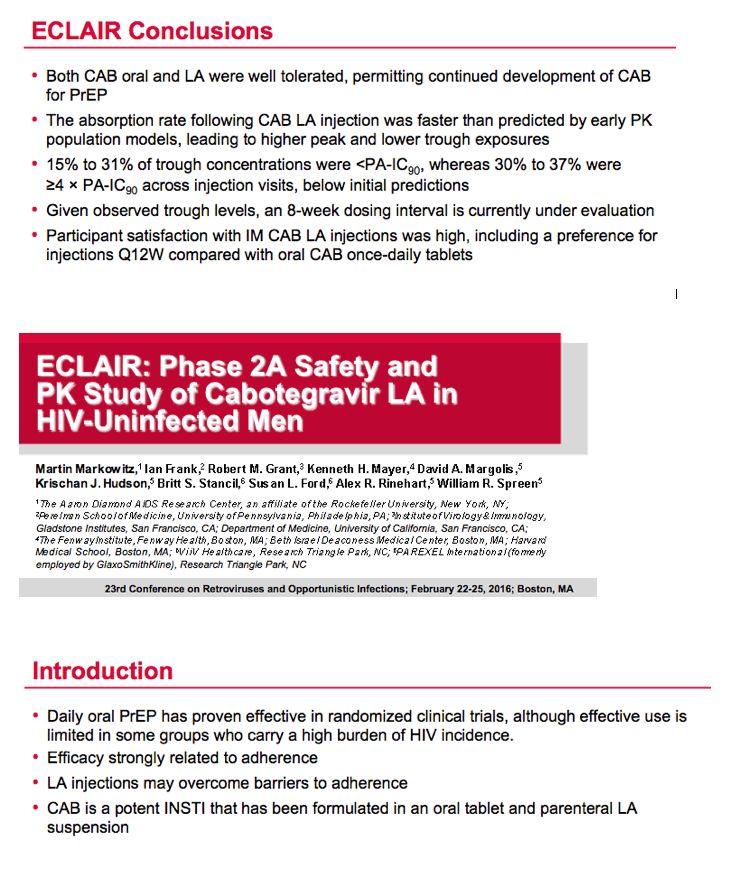
Pharmacokinetic (PK) data from the ECLAIR study of long-acting cabotegravir as preexposure prophylaxis (PrEP) in HIV-negative men suggest injections every 8 weeks rather than every 12, as in this trial, may be optimal [1]. Study participants preferred injections to once-daily tablets.
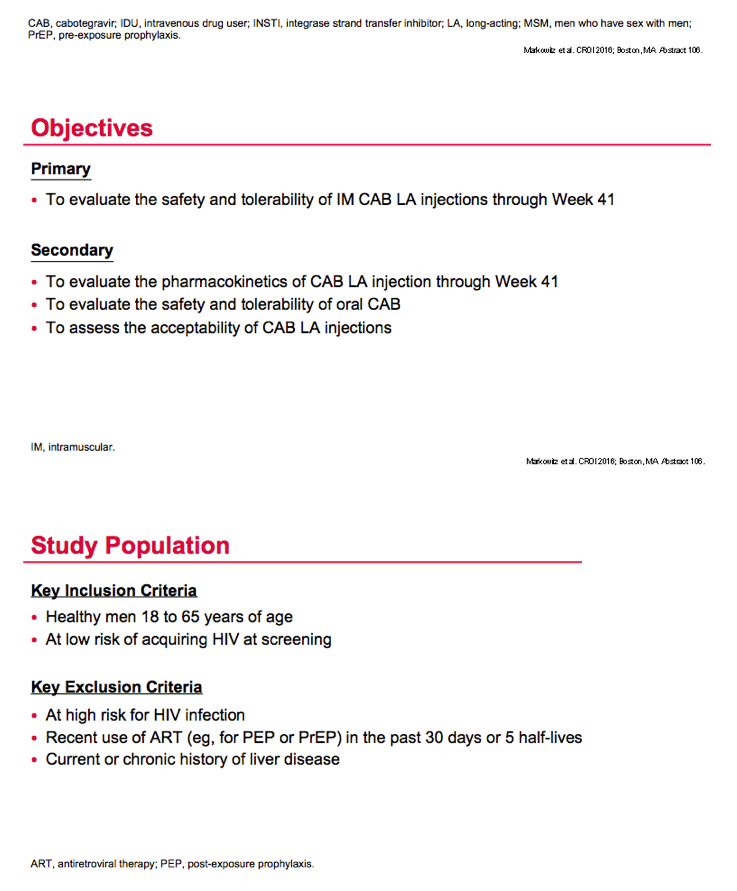
Cabotegravir is an investigational integrase inhibitor with two formulations, oral tablets and intramuscular injection. A possible HIV treatment strategy involves induction with oral cabotegravir plus other antiretrovirals and maintenance with oral cabotegravir plus rilpivirine [2] or maintenance with long-acting intramuscular cabotegravir plus rilpivirine [3]. The study presented at CROI aimed to evaluate safety and PKs of intramuscular cabotegravir injections for PrEP, and to assess the safety and tolerability of oral cabotegravir.
ECLAIR investigators recruited healthy men 18 to 65 years old with a low risk of HIV infection, defined as having at least one casual sex partner in the past 24 months. The study excluded men at high risk of HIV infection, men who recently took antiretrovirals as PrEP or postexposure prophylaxis, and men with current or chronic liver disease.
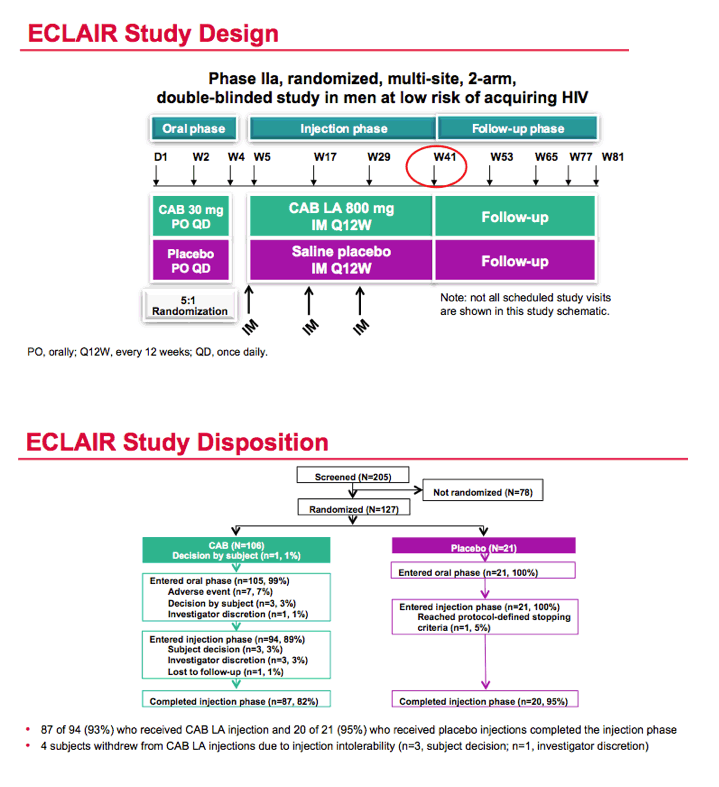
In a 5-to-1 randomization, participants took 30 mg of oral cabotegravir daily or placebo for 4 weeks followed by 1 week with no drug. Then they received 800 mg of intramuscular cabotegravir or saline placebo at weeks 5, 17, and 29 (every 12 weeks), with a primary follow-up point at week 41. Each cabotegravir dose required two 2-mL injections. Of the 106 men randomized to cabotegravir, 94 entered the injection phase and 87 (93% of 94) completed the injection phase. Of the 21 men randomized to placebo, 20 (95%) completed the injection phase.
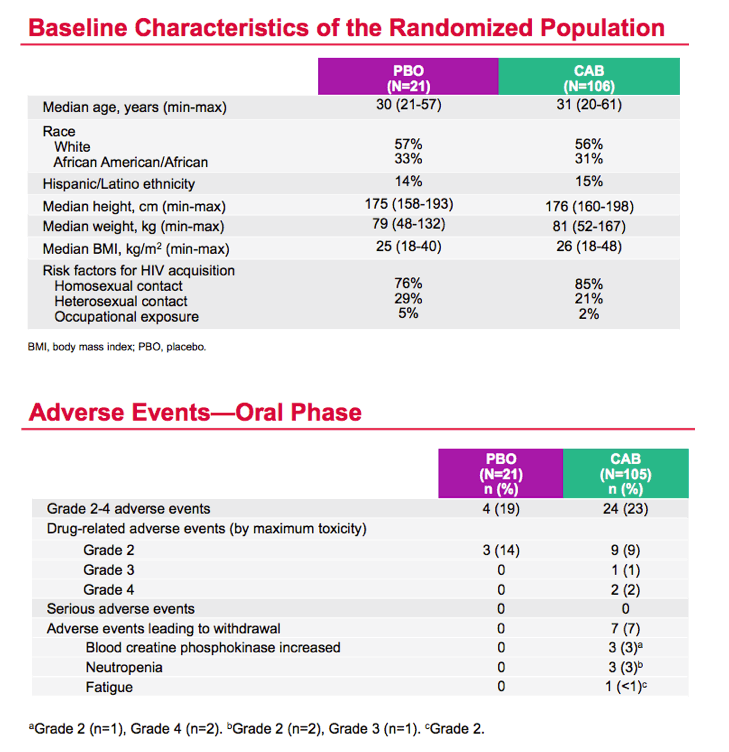
Median ages of men in the cabotegravir and placebo groups were 31 and 30, about 56% were white, one third black, and 15% Hispanic. Most men (85%) listed gay sex as their risk for HIV infection.
During the study's oral phase, grade 2 to 4 adverse events arose in 23% taking cabotegravir and 19% taking placebo. Nine grade 2 adverse events (9%) were judged drug related in the cabotegravir group, compared with 3 (14%) in the placebo group. There were 3 grade 3 or 4 drug-related adverse events in men taking cabotegravir. Seven adverse events led to withdrawal from the cabotegravir group: 3 increased blood creatine phosphokinase levels, 3 cases of neutropenia, and 1 case of fatigue.
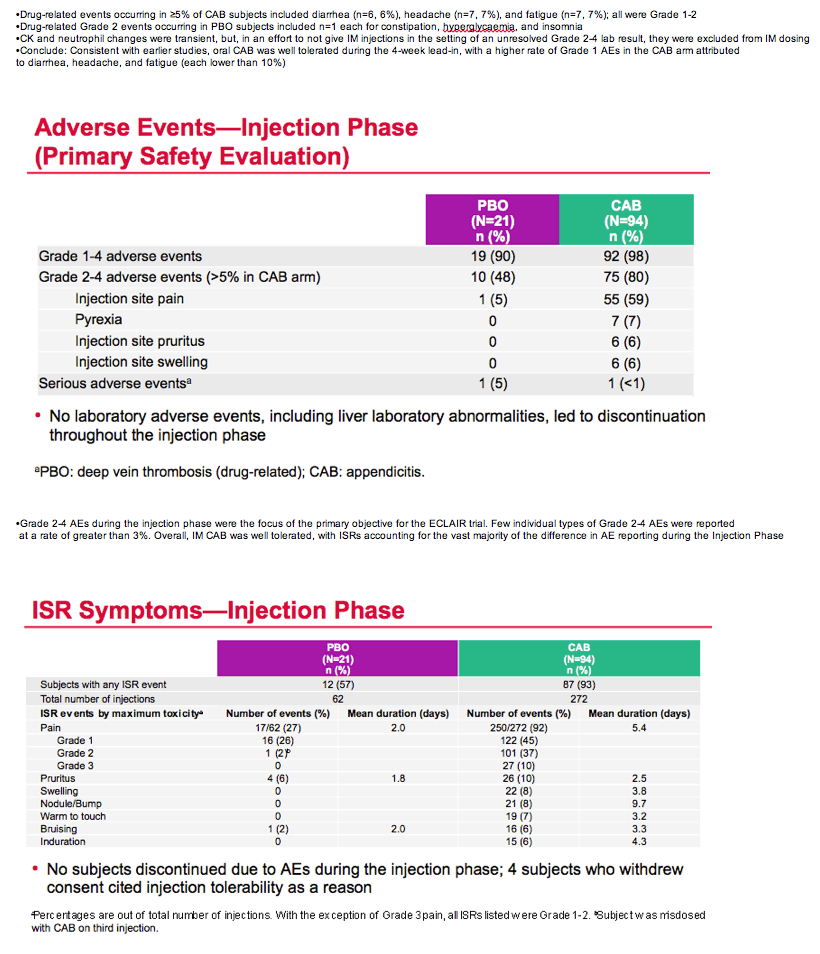
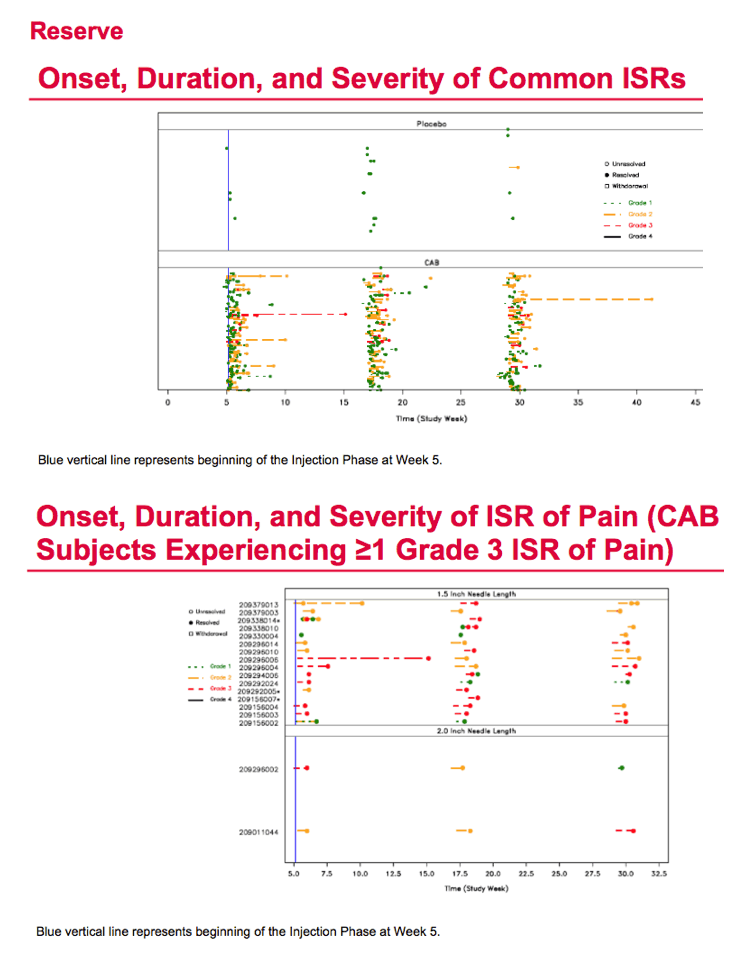
During the study's injection phase, grade 2 to 5 adverse events arose in 75 men (80%) receiving cabotegravir and 10 (48%) getting placebo. Injection site pain affected 55 men (59%) receiving cabotegravir and 1 (5%) on placebo. Other grade 2 to 4 events in the cabotegravir group were pyrexia in 7 men, injection site pruritus in 6, and injection site swelling in 6. There was 1 serious adverse event in the cabotegravir group (appendicitis). Four of 94 men who got cabotegravir shots cited injection intolerability as a reason for dropping out of the trial. Injection site pain lasted an average 5.4 days in the cabotegravir group versus 2 days in the placebo group.
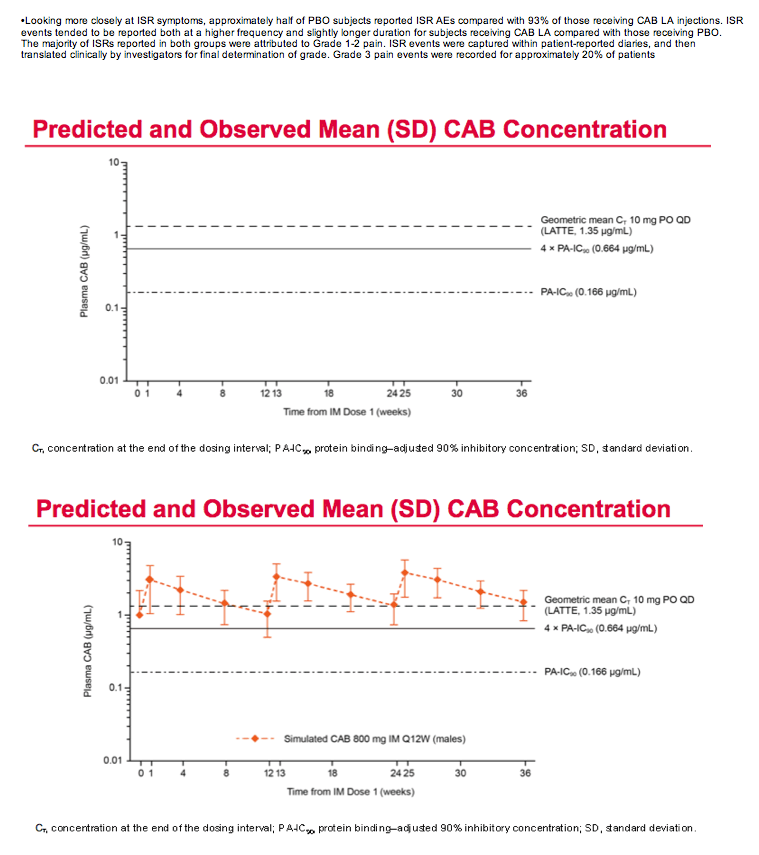
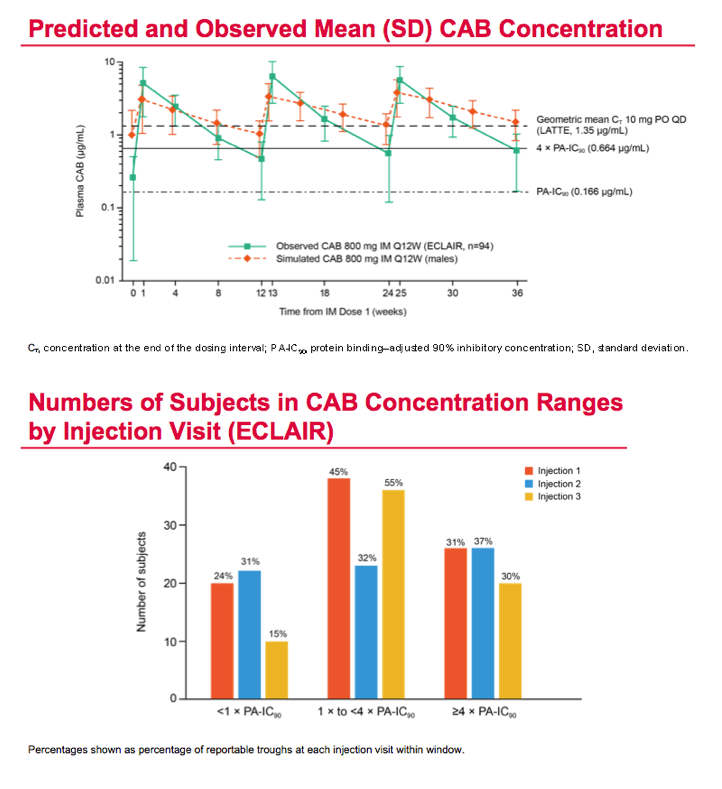
Mean cabotegravir peak concentrations proved higher than predicted from previous studies, while troughs proved lower than predicted. The mean trough after each of the three every-12-week injections lay just below the target of 4 times the protein binding-adjusted 90% inhibitory concentration (IC90) of 0.664 ug/mL. After the third injection, 15% of participants had a trough below the protein binding-adjusted IC90 (0.166 ug/mL, which probably would not protect against HIV), 55% had a trough between the IC90 and 4 times the IC90, and 30% had a trough more than 4 times above the IC990. After the first and second injections, 24% and 31% had troughs below the IC90.
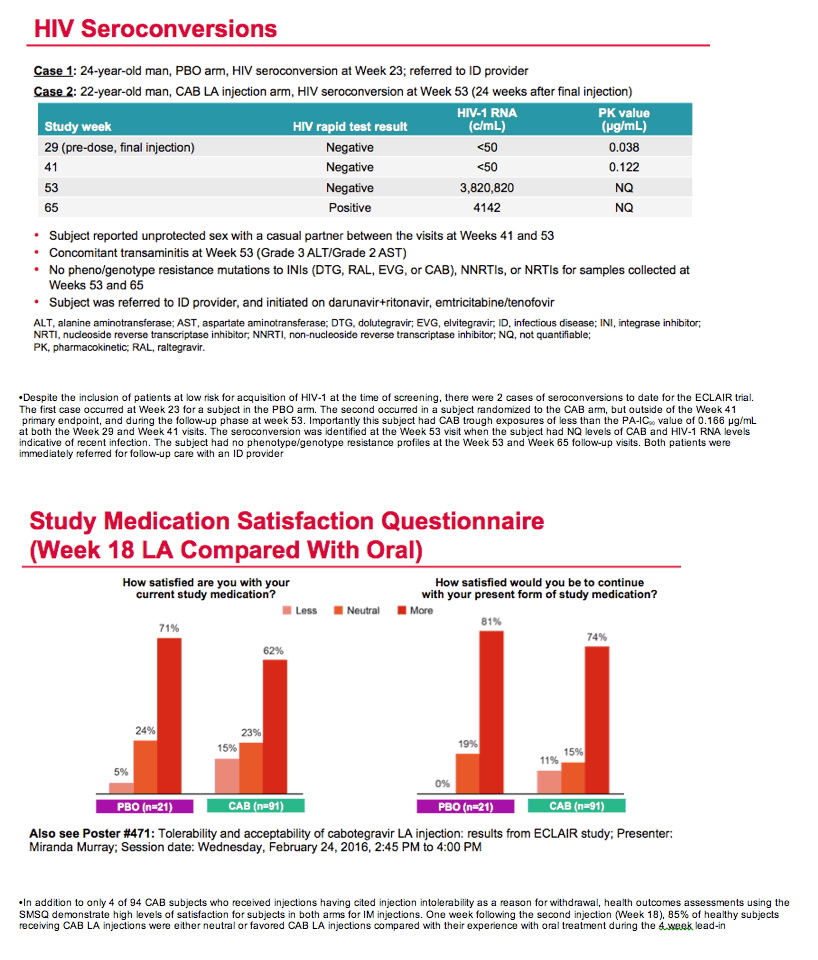
Although ECLAIR enrolled men judged to run a low risk of HIV infection, 2 men did pick up the virus, 1 in the placebo arm and 1 after three cabotegravir shots. This man tested positive 24 weeks after the final injection with no detectable cabotegravir in his blood. At the week 29 and week 41 visits, he had cabotegravir concentrations below the protein binding-adjusted IC90 of 0.166 ug/mL.
A medication satisfaction questionnaire completed 1 week after the second injection revealed that 62% of men favored cabotegravir injections over oral cabotegravir, and another 23% had no preference between the two formulations.
The researchers concluded that injected cabotegravir got absorbed faster than anticipated, and faster absorption led to higher than expected peak concentrations and lower than anticipated troughs. "Given observed trough levels," the ECLAIR team concluded, "an 8-week dosing interval [at a 600-mg dose] is currently under evaluation."
ReferencesL
1. Markowitz M, Frank I, Grant R, et al. ECLAIR: Phase 2A safety and PK study of cabotegravir LA in HIV-uninfected men. Conference on Retroviruses and Opportunistic Infections (CROI), February 22-25, 2016, Boston. Abstract 106.
2. ViiV Healthcare. Dose ranging study of GSK1265744 plus nucleoside reverse transcriptase inhibitors for induction of human immunodeficiency virus -1 (HIV-1) virologic suppression followed by virologic suppression maintenance with GSK1265744 plus rilpivirine. NLM Identifier NCT01641809. http://www.clinicaltrials.gov/ct2/show/NCT01641809
3. ViiV Healthcare. A phase IIb study to evaluate a long-acting intramuscular regimen for maintenance of virologic suppression (following induction with an oral regimen of GSK1265744 and abacavir/lamivudine) in human immunodeficiency virus type 1 (HIV-1) infected, antiretroviral therapy-naive adult subjects. NLM Identifier NCT02120352. http://www.clinicaltrials.gov/ct2/show/NCT02120352
---------------
ECLAIR: Phase 2A Safety and PK Study of Cabotegravir LA in HIV-Uninfected Men
Reported by jules Levin
CROI 2016 Feb 22-25 Boston
ECLAIR: Phase 2A Safety and PK Study of Cabotegravir LA in HIV-Uninfected Men
Reported by jules Levin
CROI 2016 Feb 22-25 Boston
|
| |
|
 |
 |
|
|