 |
 |
 |
| |
C-SWIFT RETREATMENT FINAL RESULTS: HIGHLY SUCCESSFUL RETREATMENT OF GT1-INFECTED PATIENTS WITH 12 WEEKS OF ELBASVIR/GRAZOPREVIR PLUS SOFOSBUVIR AND RIBAVIRIN AFTER FAILURE OF SHORT-DURATION ALL-ORAL THERAPY
|
| |
| |
Reported by Jules Levin
EASL 2016 April 14-17 Barcelona
Eric J. Lawitz* 1, Fred Poordad1, Julio Gutierrez1, Jennifer Wells1, Carmen Landaverde1, Joseph Reiling2, Jerry Jing Li2, Hsueh-Cheng Huang2, Michael Robertson2, Janice Wahl2, Eliav Barr2, Barbara Haber2
1TEXAS LIVER INSTITUTE, San Antonio, 2Merck & Co., Inc., Kenilworth, United States
AASLD/2014: C-SWIFT: Grazoprevir (MK-5172) + Elbasvir (MK-8742) + Sofosbuvir in Treatment-Naive Patients With Hepatitis C Virus Genotype 1 Infection, With and Without Cirrhosis, for Durations of 4, 6, or 8 Weeks (Interim
Results)......http://www.natap.org/2014/AASLD/AASLD_11.htm
EASL/2015: C-SWIFT: GRAZOPREVIR/ELBASVIR + SOFOSBUVIR IN CIRRHOTIC AND NONCIRRHOTIC, TREATMENT-NAIVE PATIENTS WITH HEPATITIS C VIRUS GENOTYPE 1 INFECTION, FOR DURATIONS OF 4, 6 OR 8 WEEKS AND GENOTYPE 3 INFECTION FOR DURATIONS OF 8 OR 12 WEEKS - (04/23/15)
AASLD/2015: C-SWIFT Retreatment (Part B): 12 Weeks of Elbasvir/Grazoprevir with Sofosbuvir and Ribavirin Successfully Treated G1-Infected Subjects who Failed Short-Duration All-Oral Therapy - (11/23/15)

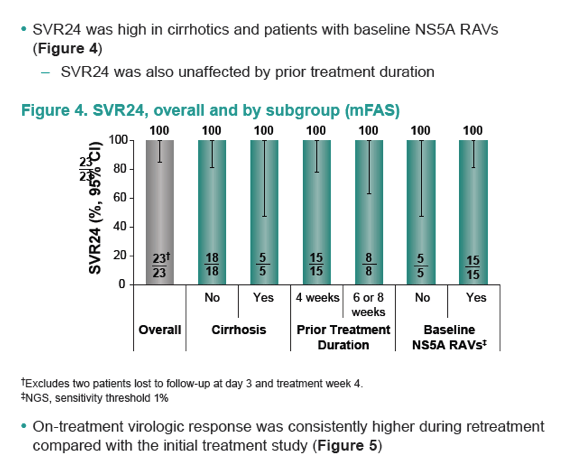
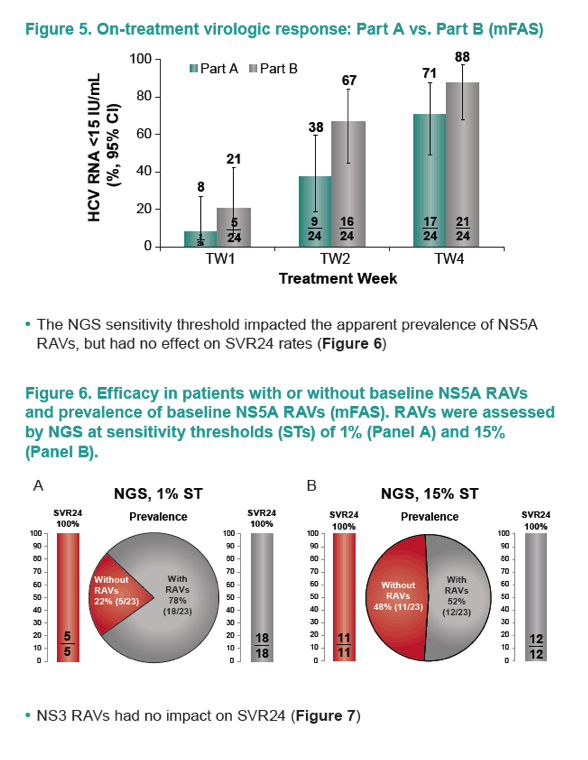
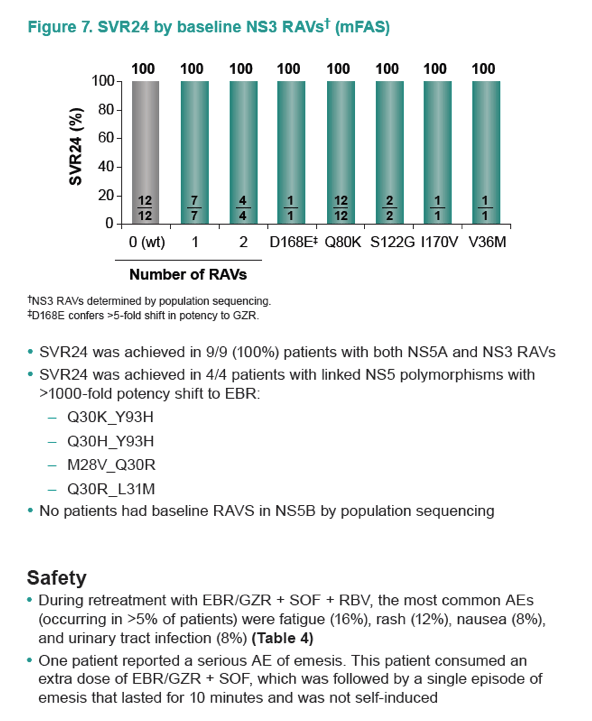

|
| |
|
 |
 |
|
|