 |
 |
 |
| |
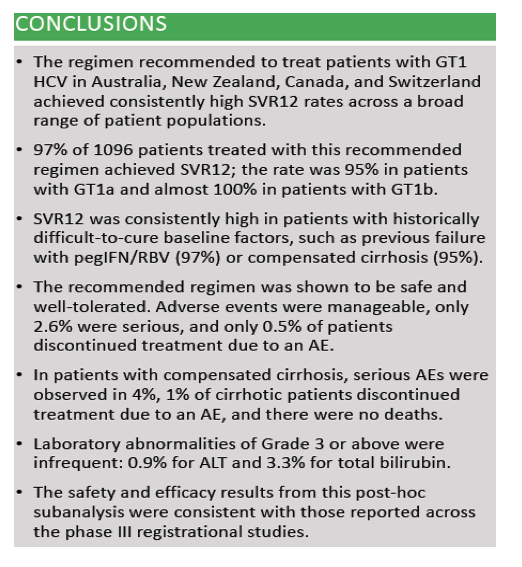
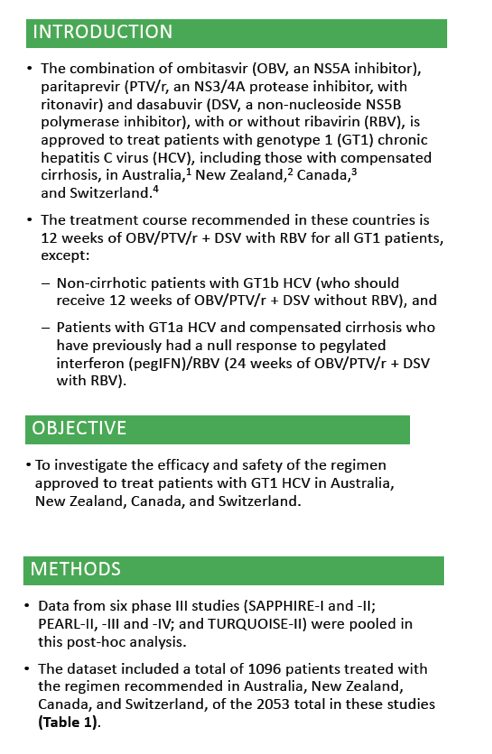
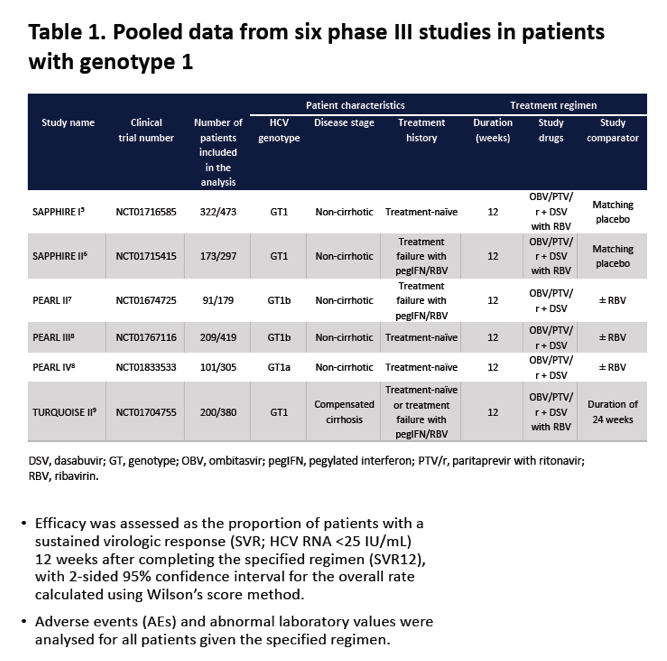
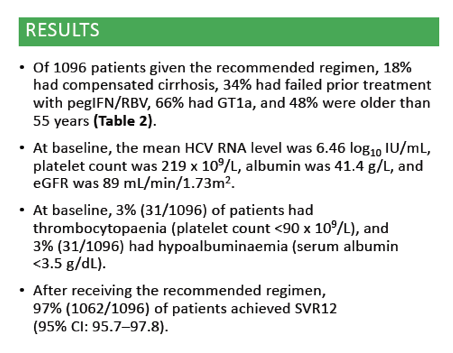
Efficacy and safety of ombitasvir/paritaprevir/ritonavir and dasabuvir, with or without ribavirin, in adults treated with the regimen approved in Australia, Canada, New Zealand and Switzerland .....Global Cohorts/ABT493+ABT530
|
| |
| |
Reported by Jules Levin
EASL 2016 April 1417 Barcelona
Edward Gane1, Simone I. Strasser2, Jordan Feld3, Jean-Francois Dufour4, Darrell Crawford5, Stuart Roberts6, Tara Satyanand7, Mudra Kapoor8, Thomas Podsadecki9, Lois Larsen9 1Auckland City Hospital, Auckland, New Zealand; 2Royal Prince Alfred Hospital, Sydney, Australia; 3Toronto Centre for Liver Disease, Toronto, Canada; 4Bern University Hospital, Bern, Switzerland; 5University of Queensland, Brisbane, Australia; 6Alfred Hospital, Melbourne, Australia; 7AbbVie, Auckland, New Zealand; 8AbbVie Pte Ltd, Singapore; 9AbbVie Inc, North Chicago, United States.
EASL: REAL-WORLD SAFETY AND EFFECTIVENESS OF OMBITASVIR/PARITAPREVIR/R ± DASABUVIR ± RIBAVIRIN IN THE GERMAN HEPATITIS C REGISTRY (clear version) - (04/15/16)
EASL: The Real-World Israeli experience of treating chronic hepatitis C (CHC), genotype 1 (GT1) patients with advanced fibrosis with paritaprevir/ritonavir/ombitasvir, dasabuvir with or without ribavirin (3D±R): a large multi-center cohort - (04/15/16)
EASL: Effect of Baseline Resistance-Associated Variants on SVR With the 3D Regimen Plus RBV - (04/18/16)
EASL: 100% SVR12 WITH ABT-493 AND ABT-530 WITH OR WITHOUT RIBAVIRIN IN TREATMENT-NAÏVE HCV GENOTYPE 3-INFECTED PATIENTS WITH CIRRHOSIS
EASL: HIGH SVR RATES WITH ABT-493 + ABT-530 CO-ADMINISTERED FOR 8 WEEKS IN NON-CIRRHOTIC PATIENTS WITH HCV GENOTYPE 3 INFECTION -
EASL: High SVR Rates With the Combination of ABT-493 + ABT-530 for 8 Weeks in Non-Cirrhotic Patients With HCV Genotype 1 or 2 Infection - (04/18/16)
EASL: Safety of ABT-493 + ABT-530 Co-Administered in Patients With HCV Genotype 1-6 Infection: Results From the SURVEYOR-I and SURVEYOR-II Studies
EASL: 100% SVR4 and Favorable Safety of ABT-493 + ABT-530 Administered for 12 Weeks in Non-Cirrhotic Patients With Genotypes 4, 5, or 6 Infection (SURVEYOR-I)
EASL: High Efficacy and Favourable Safety of ABT-493 + ABT-530 Co-Administration for 12 Weeks in HCV Genotype 1-Infected Patients With Cirrhosis (SURVEYOR-I)
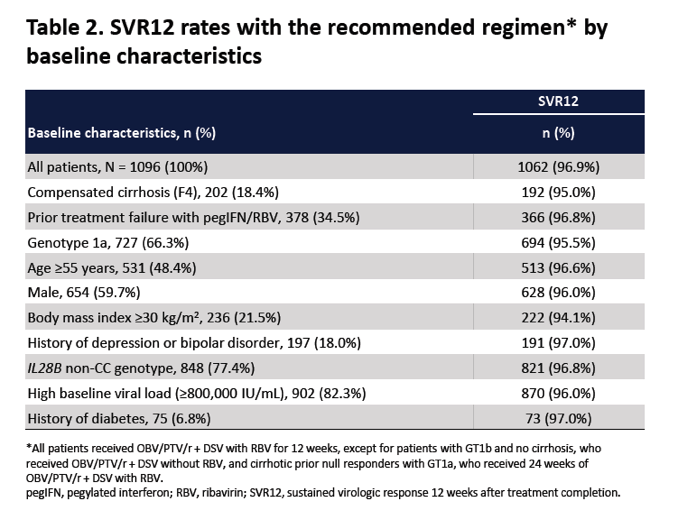
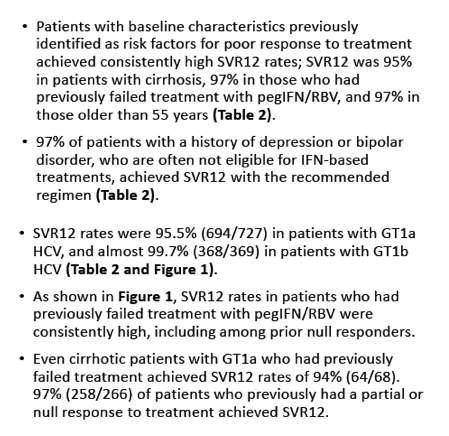
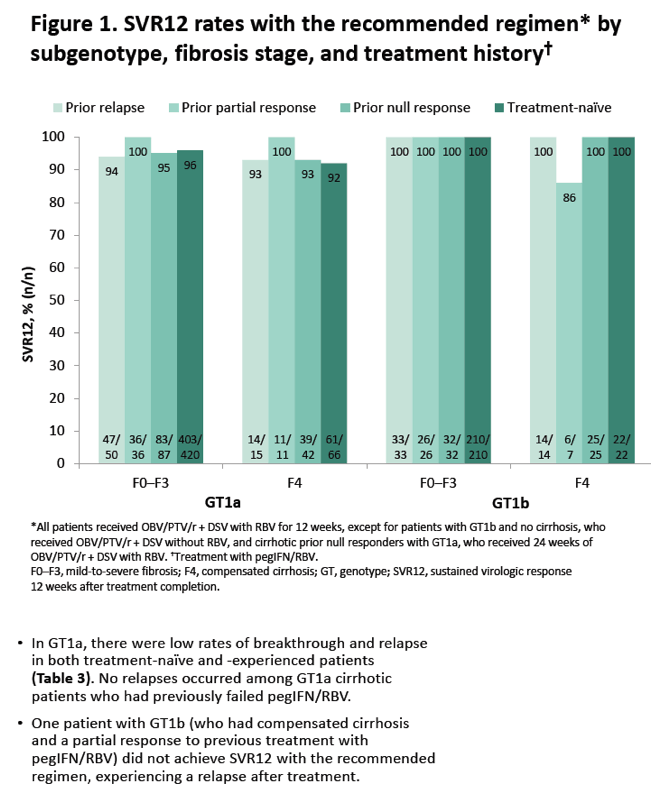
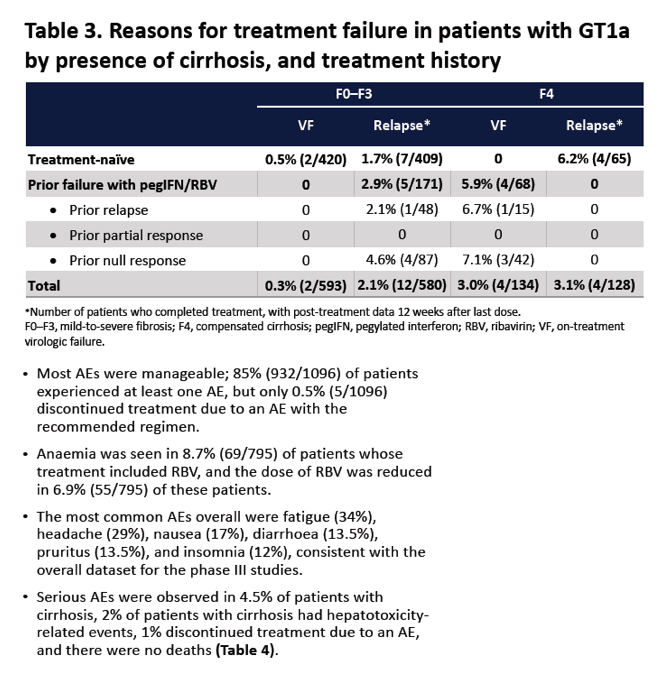
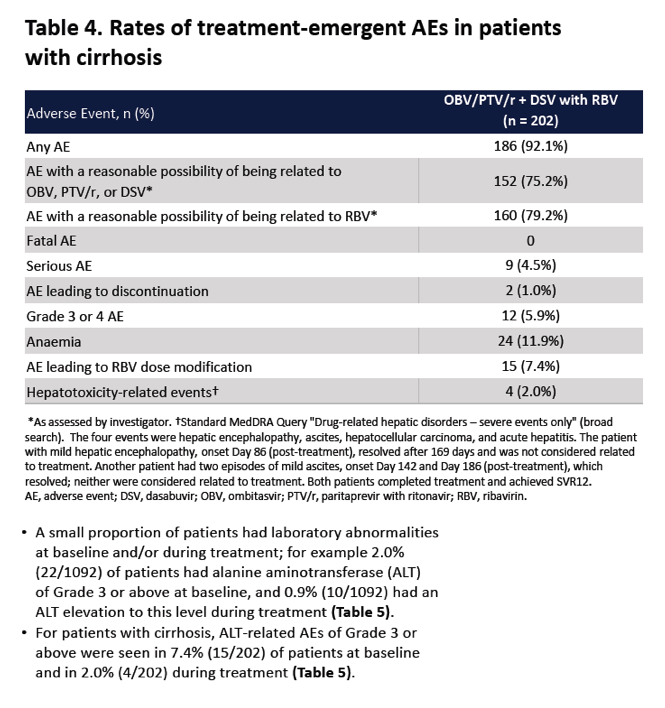
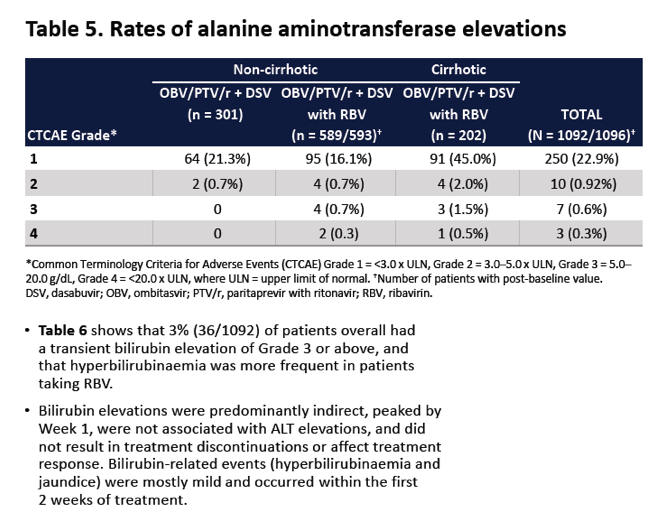
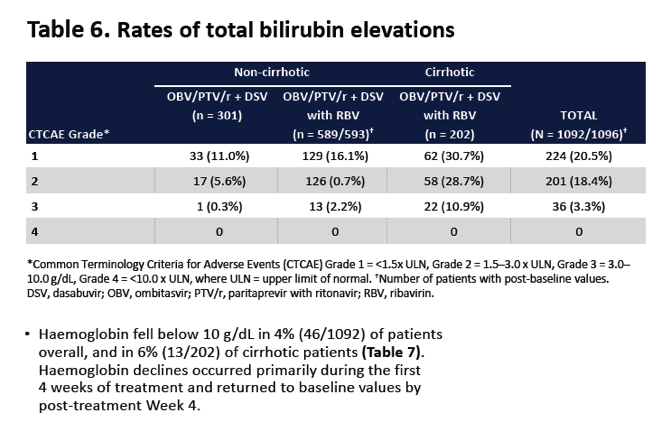
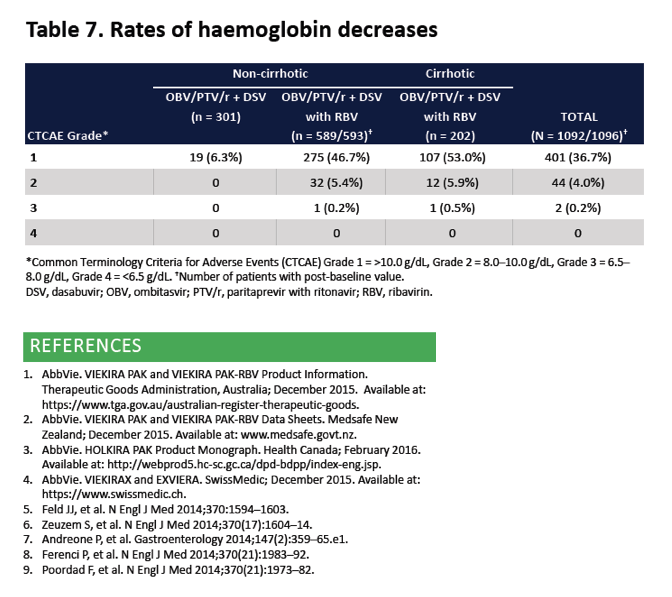
|
| |
|
 |
 |
|
|