 |
 |
 |
| |
HCV DAAs Resistance / New HCV Drugs - Direct antiviral combination treatment according to presence of baseline RAVs in NS3, NS5A and NS5B leads to high SVR rates in non-cirrhotic and cirrhotic genotype 1 infected patients
|
| |
| |
from Jules: the new 2nd/3rd generation HCV regimens change the discussion about HCV resistance / RAVs.
EASL: Resistance Analysis in 1284 Patients With Genotype 1-6 HCV Infection Treated With Sofosbuvir/Velpatasvir in the Phase 3 ASTRAL-1, ASTRAL-2, ASTRAL-3, and ASTRAL-4 Studies - (04/15/16)
EASL: HIGH EFFICACY OF ABT-493 AND ABT-530 IN HCV GENOTYPE 1-INFECTED PATIENTS WHO HAVE FAILED DIRECT-ACTING ANTIVIRAL-CONTAINING REGIMENS: THE MAGELLAN-I STUDY - (04/15/16)
EASL: High Efficacy and Favourable Safety of ABT-493 + ABT-530 Co-Administration for 12 Weeks in HCV Genotype 1-Infected Patients With Cirrhosis (SURVEYOR-I) - (04/18/16)
EASL: High SVR Rates With the Combination of ABT-493 + ABT-530 for 8 Weeks in Non-Cirrhotic Patients With HCV Genotype 1 or 2 Infection - (04/18/16)
EASL:100% SVR4 and Favorable Safety of ABT-493 + ABT-530 Administered for 12 Weeks in Non-Cirrhotic Patients With Genotypes 4, 5, or 6 Infection (SURVEYOR-I) - (04/18/16)
EASL: High Efficacy of Sofosbuvir/Velpatasvir/GS-9857 With or Without Ribavirin for 12 Weeks in Direct-Acting Antiviral-Experienced Patients With Genotype 1 HCV Infection - (04/15/16)
EASL:In a 5-day Monotherapy Trial, MK-8408 Demonstrated Potent Antiviral Activity and Improved Resistance Profile in HCV Patients With Genotypes 1, 2, and 3 Infections - (04/18/16)
AASLD/2014: MK-8408, a Potent and Selective NS5A Inhibitor With a High Genetic Barrier to Resistance and Activity Against HCV Genotypes 1-6 - (11/12/14)
EASL:High Efficacy of an 8-Week, 3-Drug Regimen of MK-3682/Grazoprevir/MK-8408 in HCV Genotype 1, 2, or 3-Infected Patients: SVR24 Data from the Phase 2 C-CREST 1 and 2 Studies - (04/19/16)
--------------
CROI: HCV resistance to the daclatasvir/sofosbuvir combination across different HCV genotypes in the real life French Cohort - (03/24/16)
EASL: Prevalence and Impact of Baseline Resistance-Associated Variants (RAVs) on the Efficacy of Ledipasvir/Sofosbuvir or Simeprevir/Sofosbuvir Against GT1 HCV Infection: HCV-TARGET Interim Analysis - (04/21/16)
EASL: Effect of Baseline Resistance-Associated Variants on SVR With the 3D Regimen Plus RBV - (04/18/16)
EASL: Retreatment of Patients who failed DAA-Combination Therapies: Real-World Experience from a Large Hepatitis C Resistance Database - (05/27/16)
EASL:EUROPEAN RAVS DATABASE: FREQUENCY AND CHARACTERISTICS OF RAVS IN TREATMENT-NA´VE AND DAA-EXPERIENCED PATIENTS - (04/29/16)
--------------------
CROI: Genotypic and Phenotypic Characterization of Clinical HCV NS5A Drug Resistance - (03/11/16)
CROI: Resistance Associated Variants: Data From the NIAID SYNERGY Trial / Harvoni-Retreating Short Duration Therapy - (03/07/16)
CROI: CROI 2016 - Themed Discussion: Rants About HCV RAVS - (03/05/16)
--------------------
Reported by Jules Levin
EASL 2016 April Barcelona
Kai-Henrik Peiffer1, Simone Susser1, Julia Dietz1, Caterina Berkowski1, Dany Perner1, Sandra Passmann1, Mirjam Raeder1, Johannes Vermehren1, Christian Lange1, Stephanie Klein1, Nina Weiler1, Stefan Zeuzem1, Christoph Sarrazin1
1Goethe-University Hospital, Medical Clinic 1,
Frankfurt, Germany
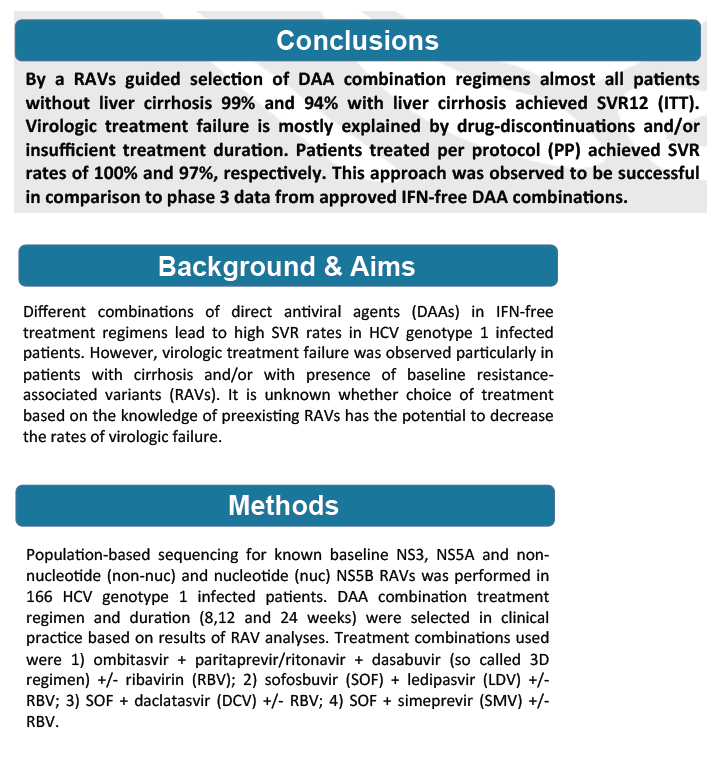
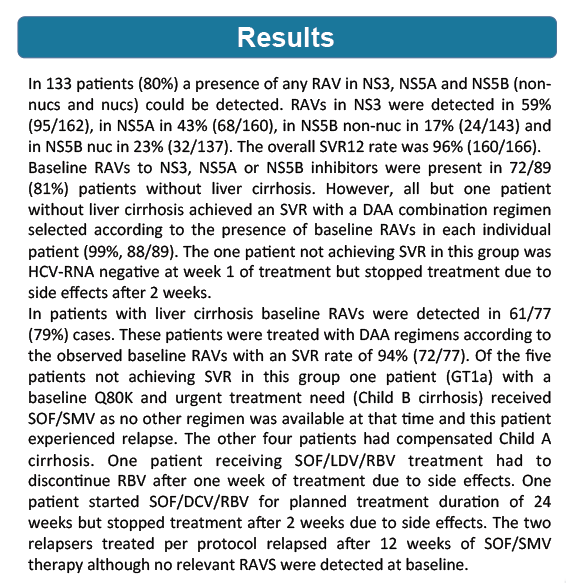
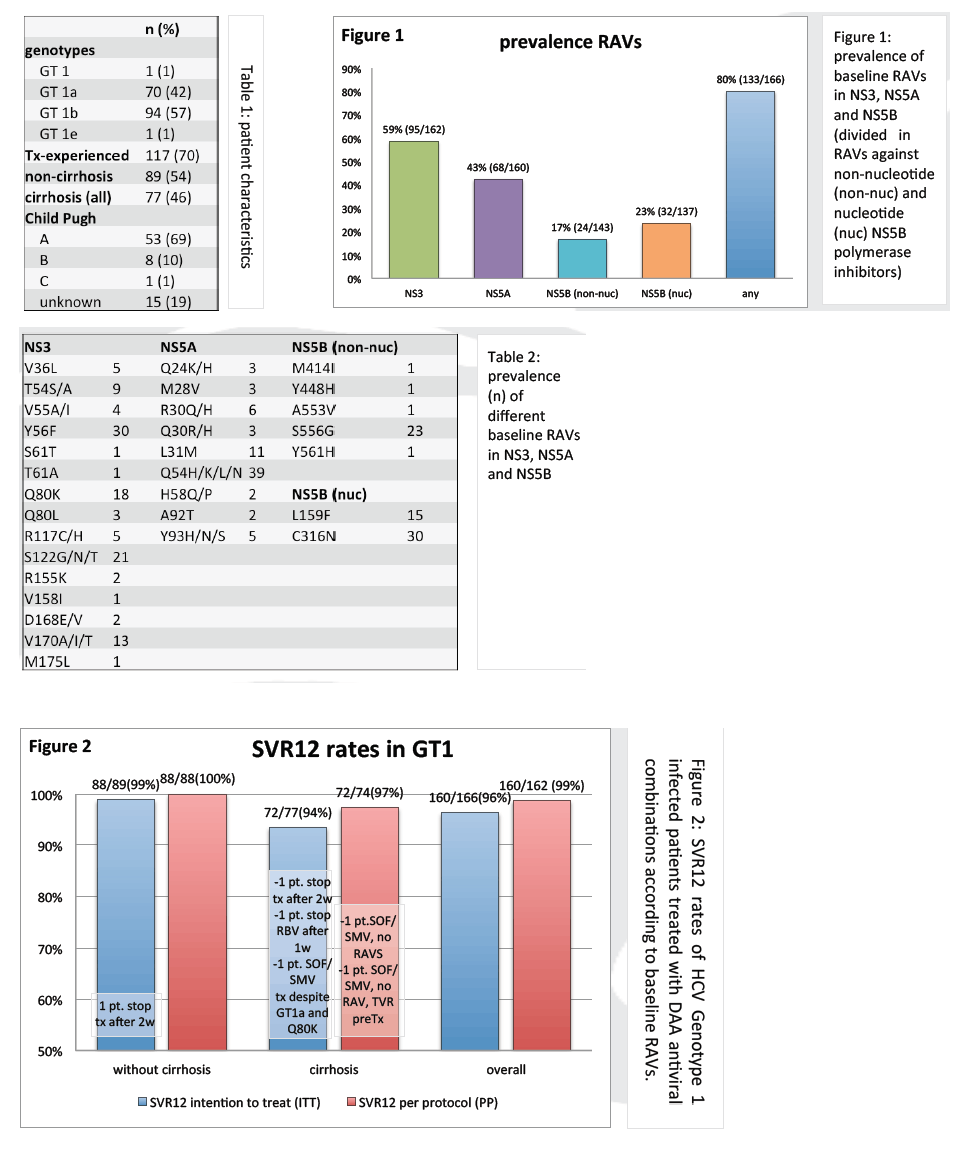
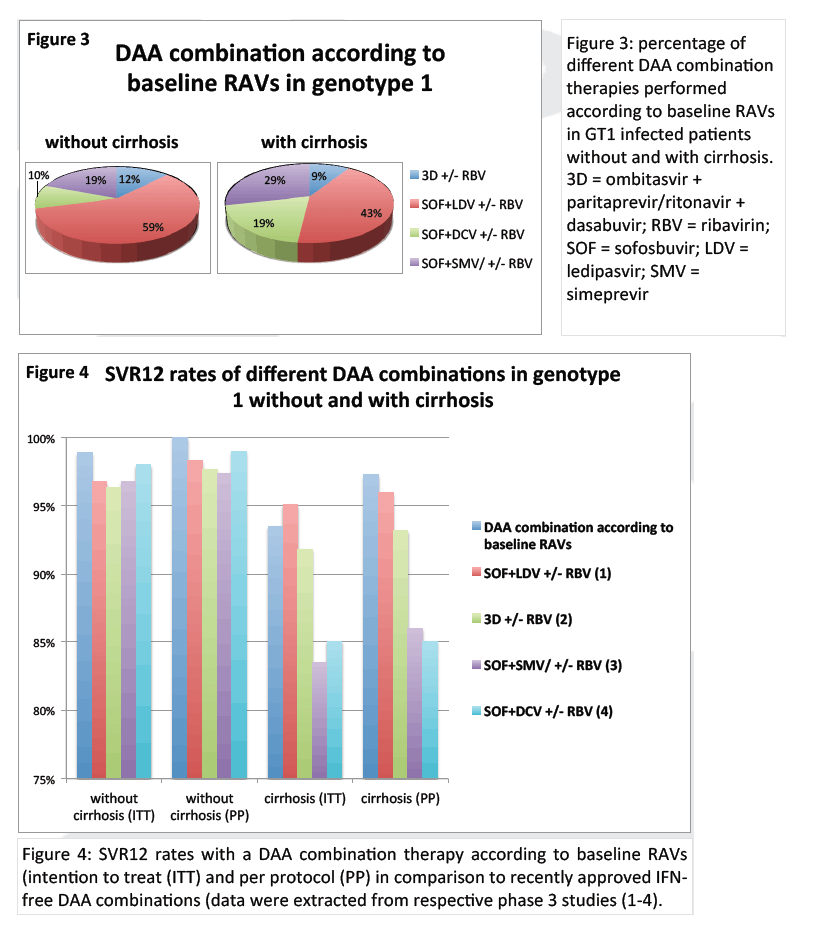
from Jules: look at Table 3
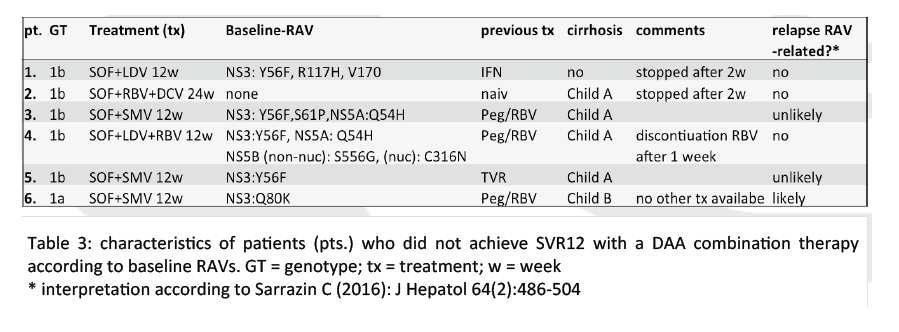
|
| |
|
 |
 |
|
|