 |
 |
 |
| |
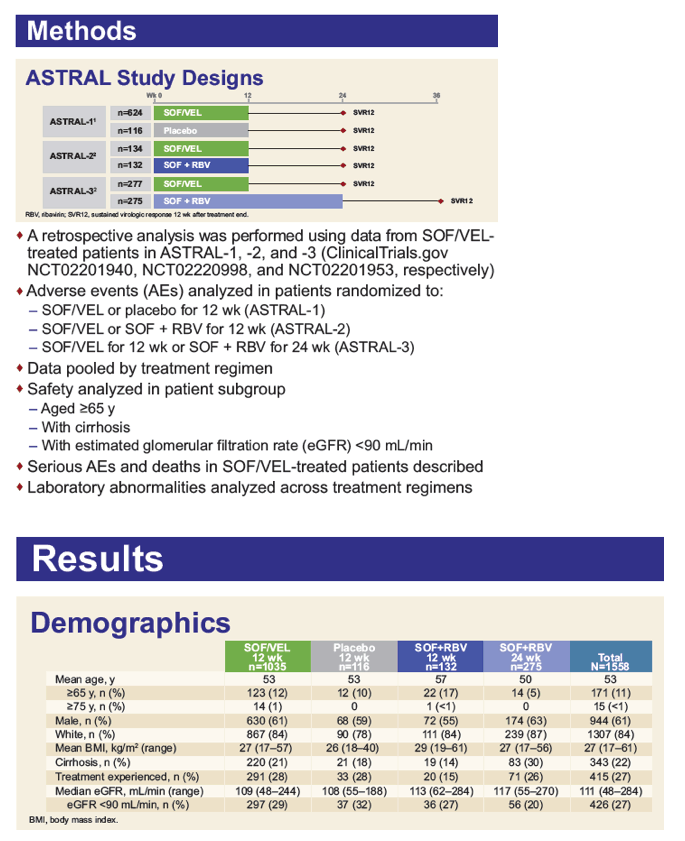
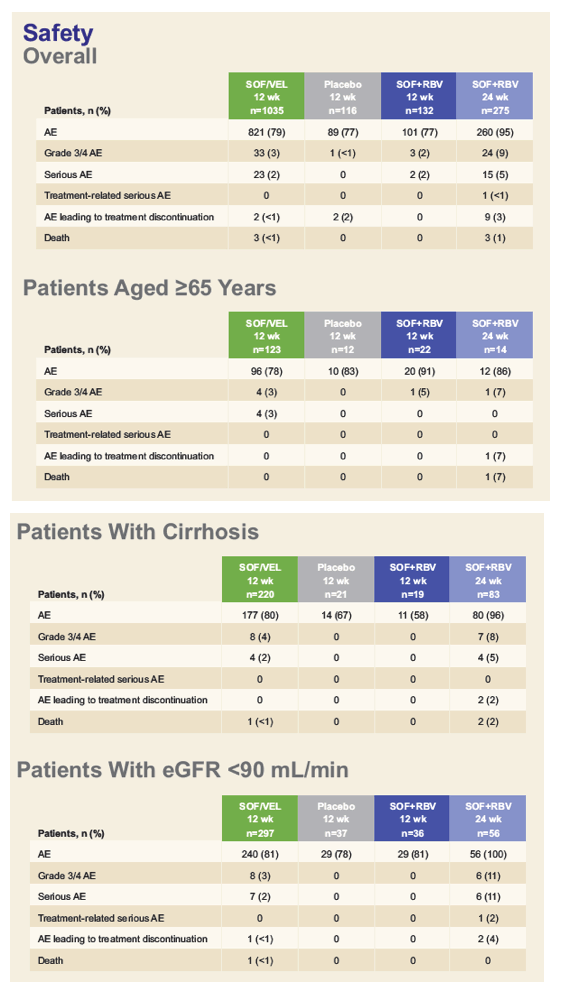
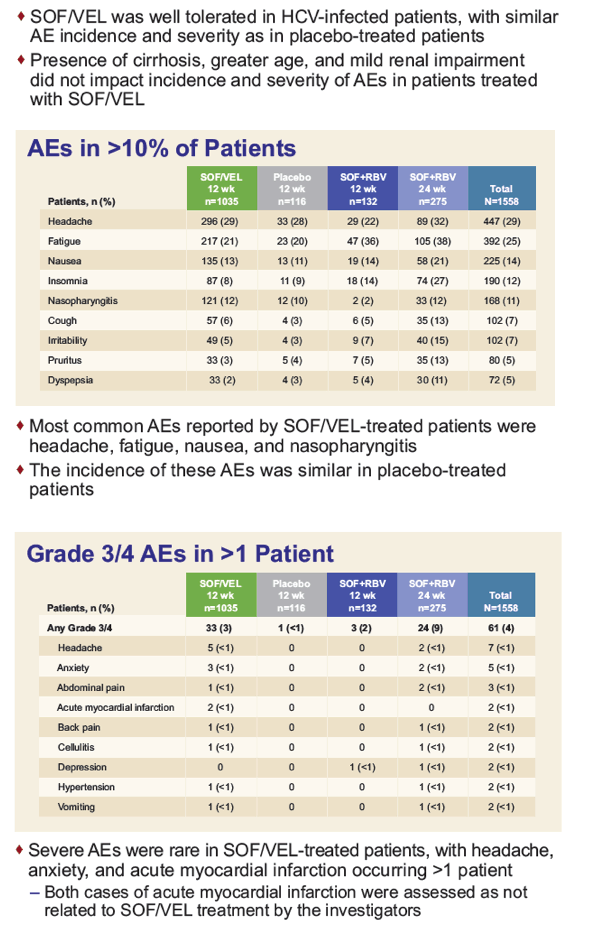
The Tolerability of Sofosbuvir/Velpatasvir for 12 Weeks in >1000 Patients Treated in the ASTRAL-1, ASTRAL-2, and ASTRAL-3 Studies: An Integrated Safety Analysis
|
| |
| |
Reported by Jules Levin
EASL 2016 April 14-17 Barcelona
Ira M. Jacobson,1 Norbert Brau,2 Stefan Bourgeois,3 Philippe Mathurin,4 Paul Thuluvath,5 W. Jeffrey Fessel,6 Stephen Ryder,7
Lin Liu,8 Xiao Ding,8 John McNally,8 Anu Osinusi,8 Diana M. Brainard,8 G. Mani Subramanian,8 Guido Gerken,9 Graham R. Foster10
1Mount Sinai Beth Israel, New York, New York, USA; 2James J. Peters VA Medical Center, Bronx, New York; 3Campus Stuivenberg, Antwerpen, Belgium; 4Centre Hospitalier Regional Universitaire de Lille and Universite Lille Nord de France; 5The Institute for Digestive Health and Liver Disease, Baltimore, Maryland, USA; 6Kaiser Permanente, San Francisco, California, USA; 7Nottingham University Hospitals NHS Trust-Queen's Medical Centre Campus, Nottingham, UK; 8Gilead Sciences, Inc., Foster City, California; 9Klinik fur Gastroenterologie und Hepatologie, Medizinisches Zentrum, Universitätsklinikum Essen, Germany; 10Queen Mary University of London, UK
|
| |
|
 |
 |
|
|