| |
"Curing" HBV - New Drugs
|
| |
| |
Curing HBV, New Drugs - [HBV at EASL 2016] - (09/09/16)
Pre-Clinical Results from ALN-HBV for the Treatment of Hepatitis B Virus Infection
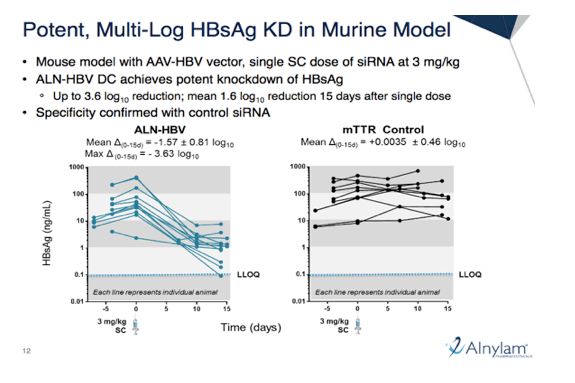
We presented new pre-clinical data with ALN-HBV, an investigational RNAi therapeutic targeting the Hepatitis B Virus (HBV) genome for the treatment of HBV infection. The ALN-HBV siRNA targets a highly conserved site across genotypes A-J, mapping to the X open reading frame, which is downstream from the most prevalent integration hotspot targeted by siRNAs from other developers. ALN-HBV is thus expected to achieve potent knockdown of HBV surface antigen (HBsAg) expressed by both covalently closed circular DNA (cccDNA) and integrated HBV DNA. Pre-clinical study results in rodent HBV models showed that subcutaneous administration of ALN-HBV led to potent and durable knockdown of HBsAg. Single doses of ALN-HBV in mice resulted in an up to 3.6 log10 and a mean of 1.6 log10 reduction of HBsAg 15 days after a single dose. Further, multiple doses of ALN-HBV in rats showed highly durable knockdown, with effects lasting up to 4 months following three weekly doses of ALN-HBV at 3 mg/kg. In addition, ALN-HBV was generally well tolerated in all rodent models.
http://www.alnylam.com/web/assets/ALN-HBV_AASLD_111515.pdf
Alnylam Initiates Phase 1/2 Clinical Trial for ALN-HBV, an Investigational RNAi Therapeutic for the Treatment of Chronic Hepatitis B Virus Infection
07.07.2016
- Company Expects to Report Initial Clinical Data in mid-2017 -
CAMBRIDGE, Mass.--(BUSINESS WIRE)-- Alnylam Pharmaceuticals, Inc. (Nasdaq: ALNY), the leading RNAi therapeutics company, today announced that it has initiated a Phase 1/2 clinical trial with ALN-HBV, a subcutaneously administered investigational RNAi therapeutic for the treatment of chronic hepatitis B virus (HBV) infection. The Phase 1/2 trial will be conducted initially in normal healthy volunteers, and, then, in chronic HBV patients. Initiation of this trial is based on encouraging pre-clinical data presented at the American Association for the Study of Liver Disease annual meeting in November 2015. The Company expects to report initial clinical data from patients in this study in mid-2017.
"We believe ALN-HBV has the potential to become a best-in-class, once-monthly, subcutaneous treatment regimen for the treatment of chronic HBV infection, including patients with both HBeAg-positive and HBeAg-negative disease" said Laura Sepp-Lorenzino, Ph.D., Vice President, Entrepreneur-in-Residence, and Program Leader for Alnylam's Hepatic Infectious Disease Strategic Therapeutic Area (STAr). "We are encouraged by the pre-clinical results with ALN-HBV demonstrating potent and durable knockdown of plasma HBV surface antigen (HBsAg) in rodent models of HBV infection. High unmet need remains for safe and effective HBV therapies with the potential to produce functional cures after a finite treatment period."
The Phase 1/2 trial of ALN-HBV is a randomized, single-blind, placebo-controlled study being conducted in three sequential parts. Part A is a single-dose study designed to enroll up to a total of 24 normal healthy volunteers (NHVs). Part B will be a single-dose study designed to enroll up to a total of 28 patients with chronic HBV infection. Part C will be a multi-dose study designed to enroll up to a total of 48 patients with chronic HBV infection. The primary objective of the study is to evaluate safety and tolerability of single and multiple subcutaneous doses of ALN-HBV. Secondary objectives include evaluation of pharmacokinetics and clinical antiviral activity for ALN-HBV as measured by its effects on serum HBsAg levels in hepatitis B envelope antigen (HBeAg) positive and negative chronic HBV patients.
ALN-HBV utilizes the company's Enhanced Stabilization Chemistry (ESC)-GalNAc-siRNA conjugate delivery platform, which enables high potency and durability with a very wide therapeutic index. Clinical results from other investigational RNAi therapeutic programs in Alnylam's pipeline that utilize ESC-GalNAc technology have demonstrated robust target gene knockdown and durability supportive of the potential for monthly, quarterly and, possibly, bi-annual subcutaneous dosing regimens, facilitating the potential for improved patient adherence.
ALN-HBV Pre-clinical Data
Pre-clinical study results in rodent HBV models showed that subcutaneous administration of ALN-HBV led to potent and durable knockdown of HBsAg. Single doses of ALN-HBV in mice resulted in an up to 3.6 log10 and a mean of 1.6 log10 reduction of HBsAg 15 days after a single dose. Further, multiple doses of ALN-HBV in rats showed highly durable knockdown, with effects lasting up to 4 months following three weekly doses of ALN-HBV at 3 mg/kg. In addition, ALN-HBV was generally well tolerated in 13-week GLP toxicology studies in rat and non-human primates.
About HBV Infection
Worldwide, 2 billion people (1 out of 3 people) have been infected with hepatitis B virus (HBV) and 400 million people have become chronically infected, including 1 to 2 million people in the U.S. An estimated 1 million people die each year from hepatitis B and its complications worldwide; about 5,000 of those are in the U.S.Worldwide, chronic infection with hepatitis causes 80% of all hepatocellular carcinoma (HCC) and more than 500,000 people die each year from this lethal cancer. About 5% of the population are chronic carriers of HBV, and nearly 25% of all carriers develop serious liver diseases such as chronic hepatitis, cirrhosis, and HCC. Current treatment options include long-term antiviral therapies that permit low-levels of virus cells to replicate leading to HBV viral persistence and affecting therapeutic outcomes. There is a significant need for safe and convenient novel therapeutics that restore host immune response through targeted HBsAg knockdown offering HBV patients the potential for 'functional cures' by eliminating virus producing cells.
About RNA
RNAi (RNA interference) is a revolution in biology, representing a breakthrough in understanding how genes are turned on and off in cells, and a completely new approach to drug discovery and development. Its discovery has been heralded as "a major scientific breakthrough that happens once every decade or so," and represents one of the most promising and rapidly advancing frontiers in biology and drug discovery today which was awarded the 2006 Nobel Prize for Physiology or Medicine. RNAi is a natural process of gene silencing that occurs in organisms ranging from plants to mammals. By harnessing the natural biological process of RNAi occurring in our cells, the creation of a major new class of medicines, known as RNAi therapeutics, is on the horizon. Small interfering RNA (siRNA), the molecules that mediate RNAi and comprise Alnylam's RNAi therapeutic platform, target the cause of diseases by potently silencing specific mRNAs, thereby preventing disease-causing proteins from being made. RNAi therapeutics have the potential to treat disease and help patients in a fundamentally new way.
|
|
| |
| |
|
|
|