| |
Monitoring of kidney function in HIV-positive patients
|
| |
| |
Download the PDF here
HIV Medicine Sept 2015
JC Yombi,1 R Jones,2 A Pozniak,2 J-M Hougardy3 and FA Post4,5
1AIDS Reference Centre, St Luc University Hospital, Catholic University of Louvain, Brussels, Belgium, 2Directorate of
HIV and Sexual Health, Chelsea and Westminster Hospital, London, UK, 3Nephrology Department, ULB Erasme
University Hospital, Brussels, Belgium, 4King's College Hospital NHS Foundation Trust, London, UK and 5King's College
London School of Medicine, London, UK
Abstract
HIV-positive patients are at increased risk of developing chronic kidney disease. Although guidelines recommend regular monitoring of renal function in individuals living with HIV, the optimal frequency remains to be defined. In this review, we discuss the renal syndromes that may be identified at an earlier stage via routine assessment of kidney function, and provide guidance in terms of the frequency of monitoring, the most useful tests to perform, and their clinical significance. Specifically, we address whether annual monitoring of kidney function is appropriate for the majority of HIV-positive patients.
Introduction
HIV infection is a risk factor for both acute kidney injury (AKI) and chronic kidney disease (CKD) [1-3]. CKD is defined by the presence of structural kidney abnormalities, an estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2, or abnormal urinary findings for more than 3 months [4]. CKD is present in approximately 17% of HIV-positive patients [5], predominantly as persistent, mild proteinuria [6, 7]. As CKD is a risk factor for kidney disease progression, cardiovascular events and death [8-10], early identification of CKD allows the institution of preventative measures to reduce the risk of complications. Consequently, regular (3-6 monthly) screening for CKD using eGFR, urine dipstick and the albumin:creatinine ratio (ACR) or protein:creatinine ratio (PCR) is recommended for HIV-positive patients in several clinical guidelines [11-13].
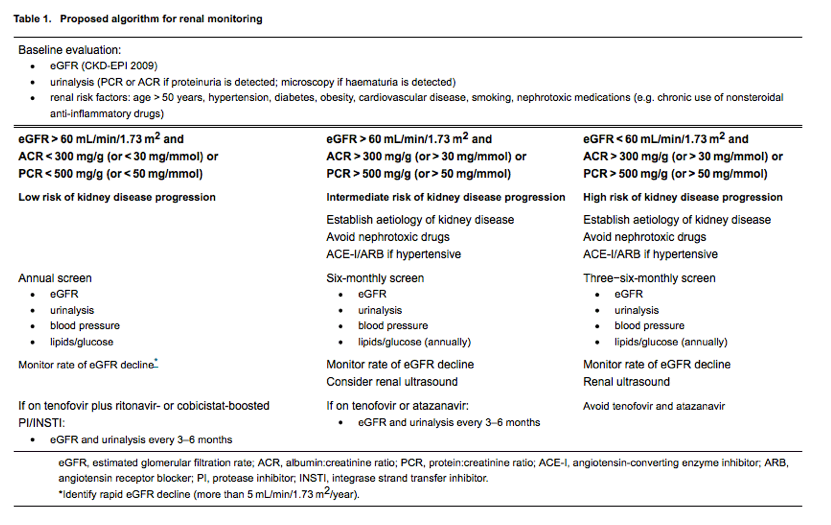
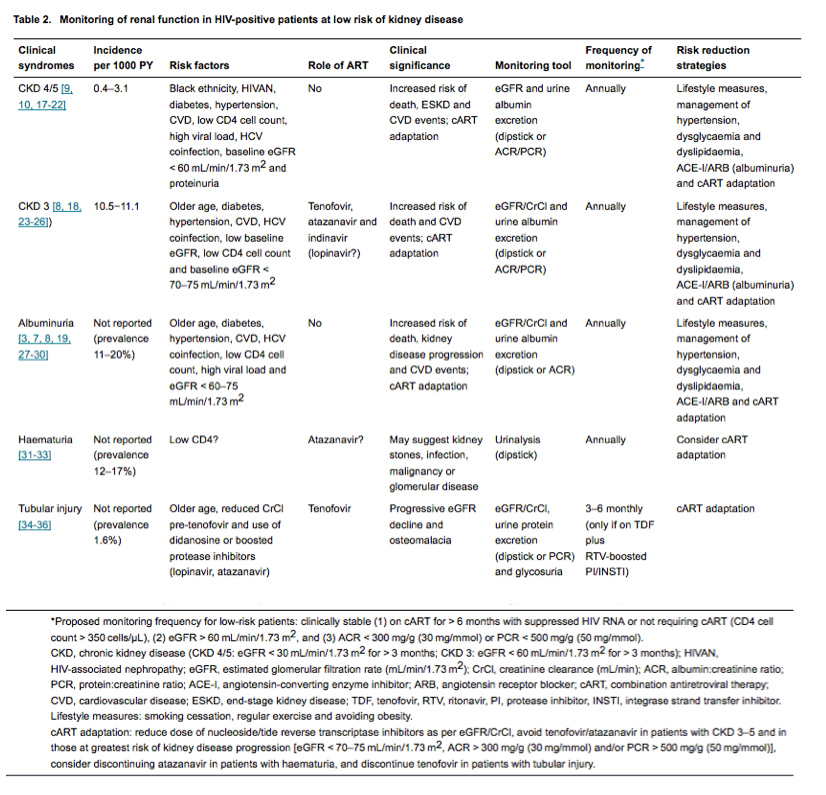
Current combination antiretroviral therapy (cART) is highly efficacious in suppressing HIV replication and preventing HIV-related illness, supporting individuals living with HIV to achieve normal or near-normal life expectancy [14, 15] and undergo clinical review bi-annually or annually. Accordingly, US guidelines suggest that CD4 cell count monitoring can be performed annually or be considered optional in stable patients with fully suppressed HIV RNA and CD4 cell counts of 300-500 and > 500 cells/μL, respectively [16]. Given that the optimal frequency of renal monitoring in the HIV context has not been studied to date, it is unclear whether an annual visit would be sufficiently frequent for the majority of HIV-positive patients to allow the early detection of CKD. In this review, we discuss the clinically important renal syndromes in HIV-positive patients in terms of their incidence and risk factors and whether an annual CKD monitoring strategy would be appropriate for stable patients on cART at low risk of kidney disease progression (Table 1). In addition, we provide guidance for harm reduction strategies in line with Kidney Disease Improving Global Outcomes (KDIGO) guidelines for the general population [4] (Table 2).
Advanced CKD [stages 4/5; estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2]
Several large HIV-infected cohort studies have reported on the incidence of advanced CKD or end-stage kidney disease (ESKD), with most estimates ranging from around 0.4 to 3.0 per 1000 person-years [9, 10, 17-22], albeit with substantially higher rates in some African-American populations [37]. Although proteinuria, older age, hepatitis C virus coinfection, hypertension, diabetes mellitus and cardiovascular events have been associated with kidney disease progression [10, 17, 19], black ethnicity and impaired kidney function at baseline (at or shortly after cohort entry or HIV diagnosis) are the strongest risk factors for developing advanced CKD [9, 10, 19]. While low CD4 cell counts and HIV viraemia are also associated with advanced CKD, antiretrovirals with nephrotoxic potential are generally avoided or discontinued in those who develop advanced CKD. This may explain why tenofovir and atazanavir have not been associated with advanced CKD or ESKD [10, 22].
The combination of eGFR and albuminuria or proteinuria allows the identification of patients at greatest risk of progression to advanced CKD [19]. The very low incidence of advanced CKD in HIV-positive patients with preserved kidney function (eGFR > 60 mL/min/1.73 m2 and urine ACR < 300 mg/g or PCR < 500 mg/g) suggests that annual monitoring would suffice in the vast majority of patients. In those with eGFR < 60 mL/min/1.73 m2 and/or ACR > 300 mg/g (or PCR > 500 mg/g), renal function should be monitored more closely and renal and cardiovascular risk factors managed more aggressively in line with KDIGO guidance for the general population [4].
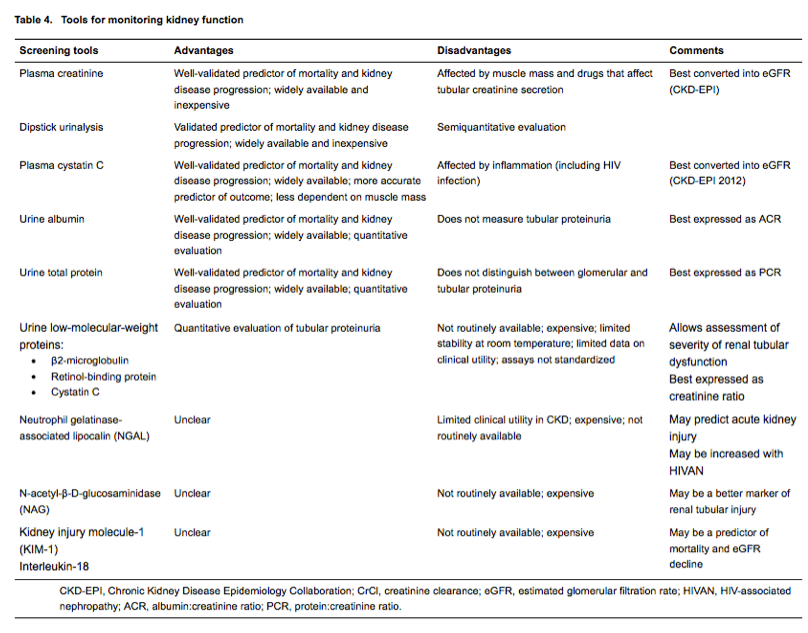
The measure most widely used to assess overall kidney function is the plasma creatinine concentration. Plasma creatinine measurements can be used to calculate the eGFR, using the Modification of Diet in Renal Disease (MDRD) or Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations, or creatinine clearance (CrCl; using the Cockcroft-Gault formula). The eGFR (CKD-EPI) provides the most accurate estimates of glomerular filtration rate when compared with gold-standard clearance measurements [38-41] and appears most predictive of clinical outcomes [42, 43], and we recommend that this formula is used to monitor renal function in HIV-positive patients. The use of the Cockcroft-Gault formula is no longer recommended [4].
Albuminuria can be measured quantitatively by immunoturbidimetry in a spot urine sample and is best expressed as the ACR [4]. The presence of albuminuria can also be detected using dipsticks which provide a semiquantitative and less sensitive estimate of the urinary albumin concentration. Albuminuria detected by either dipstick or immunoturbidimetry is an important predictor of renal and cardiovascular outcomes [8, 44, 45]. The ACR is likely to be more accurate than the dipstick [46]and allows for comparison of serial measurements. Quantification of proteinuria, using the PCR, can also be used to identify high-risk patients.
Stage 3 CKD (eGFR 30-59 mL/min/1.73 m2)
Stage 3 CKD (especially stage 3b: eGFR 30-44 mL/min/1.73 m2) is associated with an increased risk of cardiovascular morbidity and mortality [8, 23, 44] and includes the renal function threshold below which specific antiretrovirals may require dose reduction (Table 3). The overall incidence of stage 3 CKD is around 11 per 1000 person-years [18, 24, 25], with a relatively modest increase in incidence rate with drugs such as tenofovir and atazanavir (1.2-1.3-fold per year of exposure) [24, 47]. Patients who progress to stage 3 CKD typically have CKD risk factors such as diabetes mellitus, hypertension, established cardiovascular disease or exposure to drugs with nephrotoxic potential [26]. Other risk factors include older age, hepatitis C virus coinfection, low CD4 cell count, HIV viraemia, a history of AKI, the presence of mild renal dysfunction (eGFR < 70-75 mL/min/1.73 m2 and/or albuminuria) [18, 24, 26, 48], and rapid eGFR decline (≥ 5 mL/min/1.73 m2/year) [49]. In those with normal renal function (eGFR > 90 mL/min/1.73 m2), the incidence of stage 3 CKD is considerably lower (1.3 per 1000 person-years) [25, 50], and the average rate of annual eGFR decline small [18, 25, 26, 51].
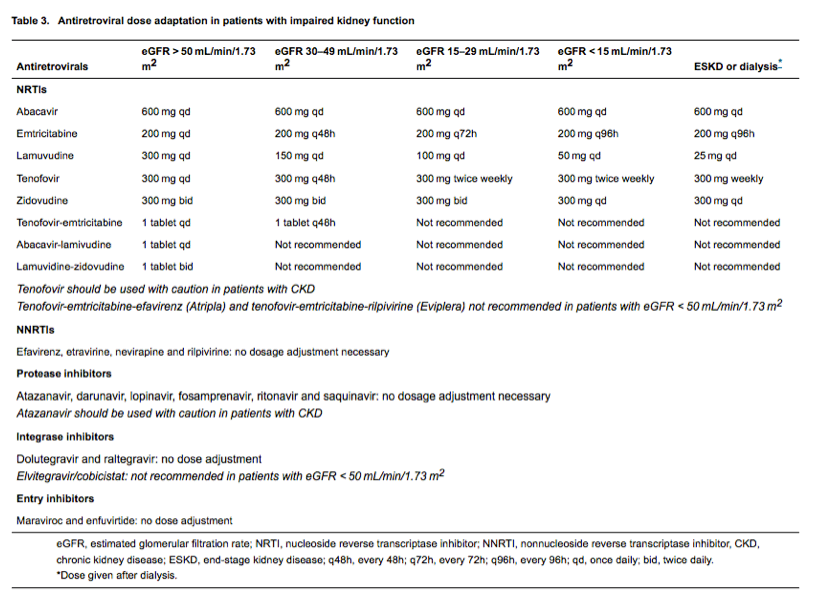
As for advanced CKD, the combination of eGFR and albuminuria allows the identification of patients with, and those most at risk of, stage 3 CKD. Annual monitoring of kidney function should suffice in the majority of patients, especially those with normal or mildly impaired kidney function (eGFR > 70-75 mL/min/1.73 m2 and ACR < 300 mg/g or PCR < 500 mg/g). Patients with stage 3 CKD should have renal and cardiovascular risk factors reviewed and managed aggressively [4], and their antiretroviral regimens and dosing schedule reviewed (Table 3); drugs such as tenofovir and atazanavir may be best avoided in those with CKD and those at greatest risk of developing CKD [52, 53].
While creatinine-based estimates can be used to detect patients with, or at risk of, stage 3 CKD [24], concern has been raised that these equations may overestimate the prevalence of CKD [42, 54], especially when using the MDRD equation [42]. In the general population, the use of plasma cystatin C measurements has been advocated to improve the predictive value of eGFR; cystatin C can either be used in addition to plasma creatinine or replace plasma creatinine in CKD-EPI equations [54]. Cystatin C-based equations appear more predictive of adverse clinical outcomes (mortality and cardiovascular events) in HIV-positive patients [27, 55, 56], even though the eGFR estimates based on cystatin C plus creatinine are generally no more accurate than the estimates based on plasma creatinine alone [38, 40]. This discrepancy may be explained by the effects of viral replication and systemic inflammation on cystatin C [57], allowing cystatin C-based equations to better identify populations at risk of adverse clinical outcomes rather than those with somewhat poorer renal function. The substantially higher cost associated with cystatin C monitoring may prove a barrier to its widespread use in clinical practice in some settings.
The Cockcroft-Gault formula has been most widely used in clinical practice to assess the need for dose reduction of renally cleared drugs in patients with impaired renal function. Okparavero and colleagues observed best concordance (79%) between plasma creatinine-based CKD-EPI-derived eGFR (compared with MDRD-derived eGFR and Cockcroft-Gault-derived CrCl) and measured GFR-assigned relevant kidney function categories as defined for drug dosing by the US Food and Drug Administration (> 80, 50-80, 30- < 50 and < 30 mL/min) in HIV-positive patients. The CKD-EPI equation also performed best in terms of correct dosing of tenofovir and emtricitabine [58].
A potentially important limitation of using plasma creatinine-based eGFR or CrCl to modify drug doses is the variable contribution of tubular creatinine secretion to plasma creatinine clearance, and the degree of inhibition of tubular creatinine secretion by specific anti-HIV drugs (including raltegravir, rilpivirine, ritonavir, cobicistat and dolutegravir) [59]. Drug-induced inhibition of tubular creatinine secretion results in higher plasma creatinine concentrations without affecting the true GFR; typically, a new CrCl/eGFR set point is reached after 2-4 weeks which is 5-20% below the pretreatment value on average [59]. The lower CrCl/eGFR estimates may result in an overestimation of the severity of renal impairment (and thus the burden of stage 3 CKD) and inappropriate dose reduction in patients with moderately impaired renal function. It remains unclear whether incorporation of cystatin C in the CKD-EPI formula is able to mitigate the effects on eGFR of drug-induced reductions in tubular creatinine secretion.
In addition to albumin or total protein, other urinary biomarkers may identify patients at increased risk of kidney disease progression. In the Women's Interagency HIV Study (WIHS) cohort, the highest tertiles of urinary interleukin-18 (IL-18) and kidney injury marker-1 (KIM-1) identified patients with greater eGFR decline, although the adjusted absolute rates of decline were very small (less than - 0.1 mL/min/1.73 m2/year); patients in the highest IL-18 tertile had a 2.2-fold increased risk of eGFR decline of more than 10% per annum [60], and a 1.9-fold increased risk of death [61]. By contrast, neutrophil gelatinase-associated lipocalin (NGAL), another biomarker of kidney injury, was not associated with renal outcomes, although others found high urinary NGAL concentrations in patients with HIV-associated nephropathy [62], a condition that predisposes to kidney disease progression [63]. The clinical utility of these biomarkers remains to be established.
Albuminuria
Urine ACR, preferably in a first morning void, is used to estimate urine albumin excretion and is categorized as < 30, 30-300 and > 300 mg/g (or < 3, 3-30 and > 30 mg/mmol; normal to mildly increased, moderately increased, and severely increased, respectively) [4, 64]. Albuminuria reflects glomerular pathology, and, while the PCR may provide an indication of glomerular protein leak, the median urine ACR to PCR ratio (APR) in HIV-positive patients with low-level proteinuria tends to be low (9.9% in a recent study [7]); PCR is thus a relatively nonspecific marker of glomerular disease, especially in patients with mild proteinuria. In one study, the incidence of proteinuria (positive dipstick analysis on two consecutive specimens) was 82 per 1000 person-years for patients not receiving tenofovir, and 132 per 1000 person-years for those on tenofovir [47]. The incidence of albuminuria in HIV-positive patients has not been reported; prevalence studies suggest that 11-20% of patients have albuminuria - mostly < 300 mg/24 h [3, 7, 27, 28]. Factors associated with albuminuria include reduced eGFR, older age, diabetes, hypertension, cardiovascular disease, hepatitis C virus coinfection, low CD4 cell count and high HIV RNA level [3, 7, 28]. Albuminuria (or proteinuria) is an independent risk factor for kidney disease progression and cardiovascular events [8, 19, 27, 44].
Albuminuria is highly prevalent in HIV-positive patients and identifies a population at increased risk of death, cardiovascular morbidity and kidney disease progression. Benefit of albuminuria reduction has not been established in HIV-positive patients. Nonetheless, cardiovascular and renal risk factors should be optimized. In patients with severe albuminuria or proteinuria (ACR > 300 and PCR > 500 mg/g, respectively), referral to a nephrologist may be indicated to establish the kidney disease aetiology. Hypertension should be managed with a renin-angiotensin-system inhibitor (i.e. an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker) as per KDIGO guidance for the general population [4]. Simultaneous assessment of ACR and PCR may be useful in distinguishing between glomerular and tubular pathology; a low APR is suggestive of tubulopathy [65]. Although the optimal frequency of albuminuria monitoring is unknown, annual assessment should suffice in the majority of patients stable on cART with ACR < 300 or PCR < 500 mg/g.
Haematuria
Haematuria is defined as the presence of three or more red blood cells per high-power field visible in at least two of three properly collected urine specimens without evidence of infection. The incidence of haematuria in HIV-positive patients has not been reported in the literature but data from prevalence studies - based on a single urine specimen - suggest that 12-17% of patients may have haematuria [31-33]. Haematuria on dipstick raises the possibility of urogenital tract infection, glomerulonephritis or urological disease including nephrolithiasis and malignancy. Glomerular disease should be considered if haematuria is accompanied by albuminuria/proteinuria, eGFR decline, hypertension and oedema, although these features may not be present in mild or early disease or specific pathologies, such as immunoglobulin A (IgA) nephropathy. The overall incidence of pathologically confirmed immune-complex kidney disease (ICKD) in HIV-infected cohorts is low (0.34 per 1000 person-years in UK Collaborative HIV Cohort Study; FA Post, King's College Hospital unpublished data); risk factors for ICKD include black ethnicity and HIV viraemia [66]. Nephrolithiasis has been reported in patients receiving (ritonavir-boosted) atazanavir-containing antiretroviral therapy, with a reported incidence of 7.3-23.7 per 1000 person-years [67-69]. One study suggested that eGFR < 60 mL/min/1.73 m2 at atazanavir initiation was a risk factor for calculi [69].
Whereas several cases of bladder cancer have been reported in older HIV-positive patients [70], data from the pre-cART era suggest that the incidence of this malignancy was unaffected by the HIV epidemic [71] and that complete urological evaluation of asymptomatic haematuria in young HIV-positive patients with normal renal function was usually nondiagnostic [72].
The incidence of clinically significant haematuria (e.g. caused by glomerulonephritis or urinary tract malignancy) appears to be very low. In patients receiving atazanavir, it is unclear whether haematuria is a useful screening tool for the early identification of nephrolithiasis. Until the latter has been clarified, it would be reasonable to limit dipstick haematuria analysis to an annual monitoring visit.
Proximal renal tubular injury
Treatment-limiting proximal tubulopathy (Fanconi syndrome) is a recognized complication of the nucleotide reverse transcriptase inhibitor tenofovir disoproxil fumarate (TDF) [34, 35, 73-75]. The true incidence of proximal tubulopathy is unknown; cohort studies and clinical trial data suggest that 0.4-1.2% of patients required TDF discontinuation because of proximal tubulopathy [35, 76]. Risk factors for TDF-associated proximal tubulopathy remain poorly defined; concomitant use of ritonavir-boosted protease inhibitors (including lopinavir/ritonavir) and reduced renal function at TDF initiation appear to be predisposing factors [35, 36, 77]. Ritonavir boosts tenofovir concentrations by approximately 30% [78]. In addition, it has been postulated that polymorphisms in the genes encoding renal tubular transporters (including those belonging to the organic anion transporter and multi-drug resistant protein families) may result in increased intracellular tenofovir exposure and thus confer susceptibility to TDF-induced proximal tubulopathy [5]. To date, only milder and typically non-treatment-limiting forms of renal tubular dysfunction have been associated with specific genetic polymorphisms [79-81]. The clinical significance of subclinical renal tubular dysfunction remains to be defined; specifically, there are no data to suggest that subclinical renal tubular dysfunction is a precursor to severe proximal tubulopathy/Fanconi syndrome.
The optimal frequency (and indeed monitoring strategy) for incident proximal tubulopathy in patients receiving TDF remains to be defined. Although cases of proximal tubulopathy were restricted to the initial 6 months of TDF exposure in the cobicistat/elvitegravir phase 3 studies [82, 83], cohort studies have reported numerous cases that occurred after more prolonged TDF exposure (median 0.6 to 2.5 years) [34, 35, 74]. The risk of proximal tubulopathy in patients who receive TDF as part of unboosted regimens, however, appears very low and these patients could be monitored annually once stable with fully suppressed HIV RNA levels. By contrast, patients who receive TDF with ritonavir- or cobicistat-boosted protease or integrase strand transfer inhibitors may require more frequent monitoring, especially during the first year. Thereafter, a monitoring frequency of 2-4 times per year seems appropriate for those with otherwise stable renal function.
Importantly, Fanconi syndrome may occur in the absence of reductions in eGFR, highlighting the need for urinalysis. The TDF clinical trials programme used treatment-emergent/worsening proteinuria, normoglycaemic glycosuria and/or hypophosphataemia, with or without reductions in eGFR/creatinine clearance, to define proximal tubulopathy. Dipstick urinalysis, eGFR and plasma glucose and phosphate measurements are widely available and easily applied in clinical practice; these may serve best for routine monitoring of patients who receive TDF. While preliminary data support the quantification of urinary low-molecular-weight proteins such as α1- or ß2-microglobulin, cystatin C or retinol-binding protein in assessing renal tubular function [84, 85], it remains unclear whether such a strategy allows the early detection of treatment-limiting proximal tubulopathy.
Guidance for clinical practice
Despite the overall increased risk of CKD, the majority of HIV-positive patients are at low risk of renal complications and it should be possible to restrict renal monitoring to annual clinic visits in stable patients with eGFR > 60 mL/min/1.73 m2 and ACR < 300 or PCR < 500 mg/g (Table 1). Typically, such patients would have fully suppressed HIV on stable cART [or preserved immunity (CD4 cell count > 350-500 cells/μL) in the absence of cART], and managed renal risk factors (e.g. hypertension, dyslipidaemia and avoidance of nephrotoxic drugs). This could include patients on non-ritonavir (or cobicistat) TDF-containing cART regimens. More frequent monitoring could be reserved for patients within the first year of starting or switching cART, especially if the cART regimens contain TDF and drugs such as cobicistat and dolutegravir that may affect creatinine secretion, those with eGFR < 60 mL/min/1.73 m2, ACR > 300 or PCR > 500 mg/g, those with immunodeficiency with uncontrolled HIV replication and those with diabetes, poorly controlled hypertension or other renal risk factors. As suggested above, for patients on TDF plus a ritonavir (or cobicistat)-boosted protease inhibitor, more frequent monitoring may be warranted beyond the first year of exposure.
In terms of monitoring tools (Table 4), creatinine measurements are inexpensive and widely available and the CKD-EPI 2009 formula should be adopted to convert these into eGFR and guide nucleoside reverse transcriptase inhibitor (NRTI) dose reductions, albeit with caution in those with extremes of body mass index (BMI) and age. Similarly, urine dipsticks are widely available and cheap and provide semiquantitative information about proteinuria, haematuria and glycosuria. The ACR, PCR and APR provide more specific and more accurate information on the amount and type of proteinuria. PCR provides information on, but does not distinguish between, glomerular and tubular dysfunction, and may thus be the preferred test for patients on TDF. By contrast, ACR may be a better predictor of the risk of kidney disease progression and identifier of patients who may benefit from renin-angiotensin-system inhibition. The role of cystatin C and quantification of low-molecular-weight proteins for the purpose of monitoring remains to be defined.
Harm reduction strategies, including smoking cessation, achievement of a healthy BMI, management of hypertension, diabetes and dyslipidaemia, and avoidance or judicious use of agents with nephrotoxic potential, are paramount in reducing the risk of kidney disease progression, cardiovascular events and death in patients with CKD. Patients with advanced CKD and unexplained rapid eGFR decline, severely increased ACR or PCR (especially with high APR) or persistent haematuria should be referred to a nephrologist for further evaluation. Those with clinical presentations suggestive of proximal tubulopathy or kidney stones should discontinue TDF and atazanavir, respectively, if alternative aetiologies have been excluded.
|
|
| |
| |
|
|
|