| |
Aging HIV+ / Kidney disease
|
| |
| |
Download the PDF here
Download the PDF here
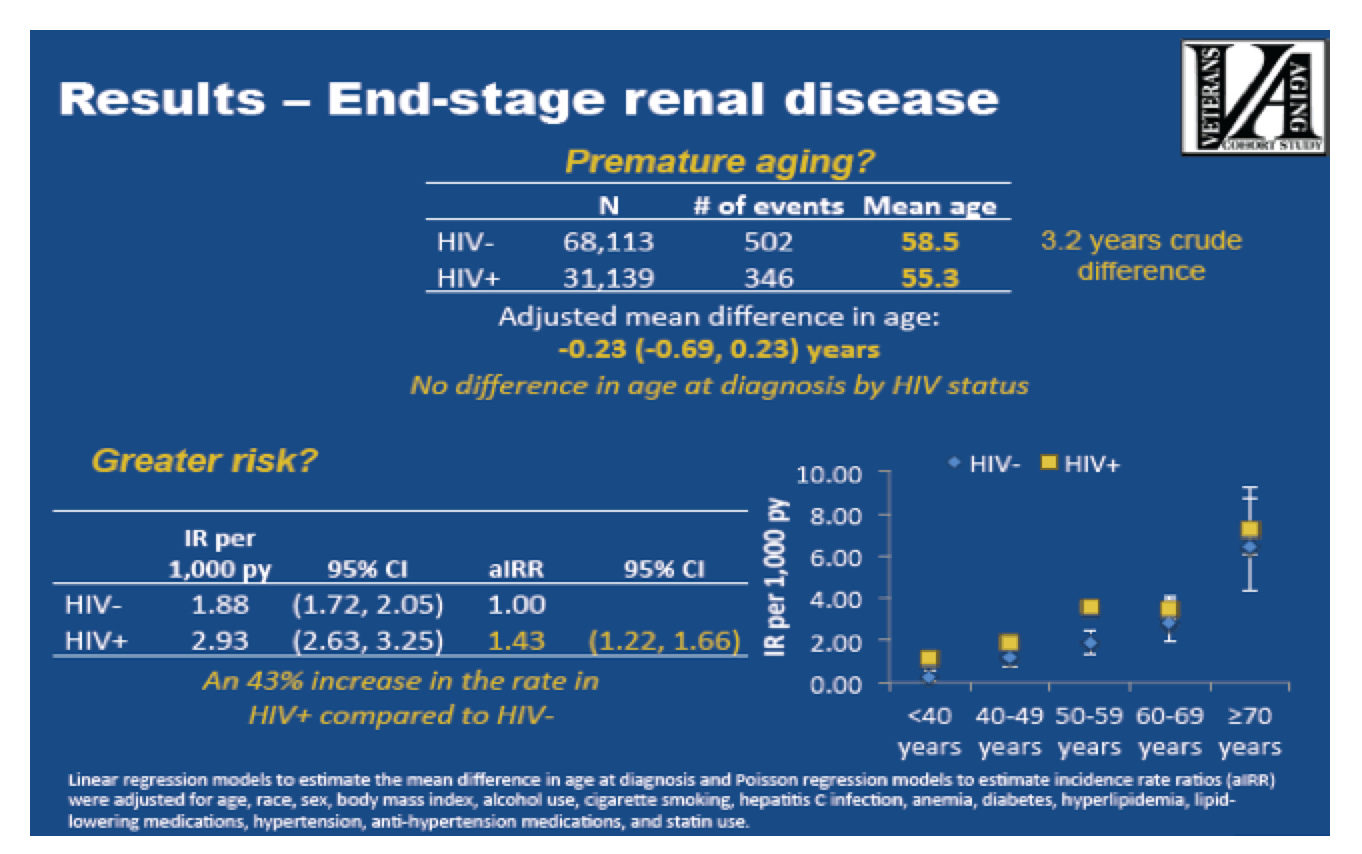
from Jules: we are facing a serious problem for older aging HIV+ with increasing comorbid conditions & disabling capacity to functions - this includes kidney disease & of course a predicted epidemic & great risk for falls & fractures. I have reported a lot on heart disease, bone disease, cancers, brain & cognitive dysfunction - all of these will be accelerated & more intensified in HIV+ & will result in a lot of disabled patients with severely reduced capacity to perform daily independent living activities. HERE is a report on kidney disease. The study below is from 2013 & looks at a relatively younger patient group with HIV. This link to CROI 2013 report looks at patients on average 55 years old & found a 43% greater risk for End Stage Renal Disease among HIV+. Again as I predict an explosion in comorbidities & increased deaths among older HIV+ over 65 years old, with an epidemic ij falls & fractures - there will I predict also be a serious development with advancing kidney disease. HCV & IDU, cocaine & heroin use or history of this - can cause kidney disease this successful HCV treatment may reduce the risk for advancing kidney disease....http://www.natap.org/2016/EASL/EASL_104.htm.......
this study from Marty Markowitz at CROI this year discusses IDU, HCV, inflammation....http://www.natap.org/2016/CROI/croi_233.htm
Alarmingly High Comorbidity Rates Among HIV+ - (09/24/15)
Comorbidities of Patients with Human Immunodeficiency Virus (HIV) in the USA - a Longitudinal Analysis of Prevalent HIV Patients Over 11 Years - (09/24/15)
2 studies associated immune activation markers/inflammation with advancing kidney disease in HIV
CROI: Kidney Dysfunction and Markers of Inflammation in the Multicenter AIDS Cohort Study - (03/06/15)
434 HIV+ men, all on antiretroviral therapy and 80% virally suppressed, and 200 HIV- men had available GFR determinations and inflammatory biomarker levels. HIV+ men had higher levels of cystatin C (median: 0.79 versus 0.75 mg/dl) and uPCr (median: 98 versus 66 mg/g).
CROI 2016: Activation and Senescence Markers in HIV Patients With Chronic Kidney Disease: "A higher CIADIS score (Higher IA and IS levels) [immune activation, senescence markers] was independently associated with advanced CKD. Although other factors may contribute to kidney damage, persisting T-cells activation and senescence could induce inflammation and thus play a direct role even in successfully treated patients."
eGFR was ≥90, 60-89 and <60 in 68 %, 28% and 4% of patients, respectively......The proportion of all comorbidities was higher among patients with an eGFR <60 (p<0.01), except for hypertension and degenerative CNS disorders. Patients with an eGFR <60 were also more likely to present two or more comorbidities (57% vs. 29%, p<0.01).....The CIADIS score was determined with CD4 and CD8 activation (DR+), maturation (T naive, memory) and senescence (CD57+CD28-) markers....In univariable analysis: an increase of the CIADIS score was significantly associated with an eGFR<60, (OR=1.2; 95% CI 1.0-1.5; P=0.04) and eGFR<90 (OR=1.1; 95% CI 1.0-1.2; P<0.01). After adjustment, the CIADIS score remained significantly associated with an eGFR<60 (OR=1.3; 95% CI 1.0-1.6; P=0.03) only. Among 221 patients with an eGFR ≥90 and available uPCr, 9% had an early kidney dysfunction.
Looking at the different immune markers used to construct the CIADIS score separately, patients with an eGFR <60 compared to those with and eGFR >=60 had higher percentages of CD4 T cells expressing CD4+ DR (16% vs. 14%, p=0.03) and lower percentages of CD8+ naïve T cells (30% vs. 39%, p=0.03), respectively.
Patients with an eGFR <90 compared to those with an eGFR >=90 had higher percentages of CD4 T cells expressing CD4+ CD57+CD28- (4% vs. 2%, p<0.01), CD8 T cells expressing CD8+ CD57+CD28- (28% vs. 26%, p<0.01) and lower percentages of CD8+ naïve T cells (37% vs. 40%, p<0.01), respectively (Data not shown).
The distribution of the CIADIS score (combining the above described markers) according to eGFR stages is depicted in Figure 1: the lower the eGFR, the higher the CIADIS score.
Poster pdf attached
------------------------------
Kidney Function 101....http://www.natap.org/2016/newsUpdates/021116_01.htm
from CROI 2013:
http://www.natap.org/2013/CROI/croi_62.htm
-----------------------
End-stage renal disease and dialysis in HIV-positive patients: observations from a long-term cohort study with a follow-up of 22 years
HIV Medicine 2013
M Bickel,1 W Marben,2 C Betz,2 P Khaykin,1 C Stephan,1 P Gute,3 A Haberl,1 G Knecht,4 T Wolf,1 HR Brodt,1
H Geiger,2 E Herrmann5 and O Jung2
1Department of Infectious Disease, Goethe University, Frankfurt/Main, Germany, 2Department of Nephrology, Goethe
University, Frankfurt/Main, Germany, 3Infektiologikum Friedensstrasse, Frankfurt/Main, Germany, 4Infektiologikum
Stresemannallee, Frankfurt/Main, Germany and 5Institute of Biostatistics and Mathematical Modeling, Department of
Medicine, Goethe University, Frankfurt/Main, Germany
"The prevalence of ESRD found in our cohort was slightly lower compared with those of other European HIV-infected cohorts, which have been found to range from 0.31 to 0.5% [26, 27]. This prevalence appears low, especially when compared with studies conducted in cohorts with higher rates of Black patients [7, 18]. Nevertheless, in a comparison with data from the German ESRD Registry [29], the prevalence (0.19 vs. 0.08%, respectively) and incidence (51.5 vs. 21.3 per 100 000 PYFU, respectively) of ESRD in the last time period were more than two times higher in HIV-infected patients than in HIV-negative individuals. The HIV-infected patients were more than 25 years younger compared with patients from the general German population at the time at which ESRD was diagnosed (median age 42.3 vs. 70 years, respectively) [29], underlining a higher risk for ESRD in HIV-infected patients. This difference compared with the general German population is not solely explained by a higher proportion of Black patients in the HIV-infected cohort, because the prevalence (0.17 vs. 0.08%, respectively) and incidence (43.4 vs. 21.3 per 100 000 PYFU, respectively) of ESRD for Caucasian HIV-infected patients were still higher compared with the German ESRD Registry [29]. This finding is contrary to results from a North American study demonstrating that the incidence of ESRD among Caucasians with HIV infection was similar to that among Caucasian patients without HIV infection [16]. This difference might be explained by the finding that most Caucasian patients with ESRD in our cohort were IDU and HCV-coinfected, whereas in most North American studies IDU and HCV coinfection were less prevalent in Caucasian patients with ESRD [19, 21, 28].
Accordingly, HCV and IDU were both identified as risk factors for ESRD in the univariate analysis of our cohort. HCV coinfection has been associated with an increased incidence and accelerated progression of chronic kidney disease (CKD) [19, 23, 30, 31]. Thus, a role in the development of ESRD as the terminal stage of CKD seems plausible. However, in the multivariate analysis, IDU but no longer HCV coinfection was significantly associated with ESRD. Due to the co-linearity of HCV coinfection and IDU, these risk factors can hardly be separated since 94.8% of IDUs were also HCV antibody positive, not only in our cohort [19, 22, 25, 27]."
Abstract
Objectives
Renal disease is a common and serious complication in HIV-infected patients.
Methods
A retrospective cohort analysis for the period 1989-2010 was carried out to determine the prevalence, incidence and risk factors for end-stage renal disease (ESRD). ESRD was defined as initiation of renal replacement therapy. Three time periods were defined: 1989-1996 [pre-highly active antiretroviral therapy (HAART)], 1997-2003 (early HAART) and 2004-2010 (late HAART).
Results
Data for 9198 patients [78.2% male; 88.9% Caucasian; cumulative observation time 68 084 patient-years (PY)] were analysed. ESRD was newly diagnosed in 35 patients (0.38%). Risk factors for ESRD were Black ethnicity [relative risk (RR) 5.1; 95% confidence interval (CI) 2.3-10.3; P < 0.0001], injecting drug use (IDU) (RR 2.3; 95% CI 1.1-4.6; P = 0.02) and hepatitis C virus (HCV) coinfection (RR 2.2; 95% CI 1.1-4.2; P = 0.03). The incidence of ESRD decreased in Black patients over the three time periods [from 788.8 to 130.5 and 164.1 per 100 000 PY of follow-up (PYFU), respectively], but increased in Caucasian patients (from 29.9 to 41.0 and 43.4 per 100 000 PYFU, respectively). The prevalence of ESRD increased over time and reached 1.9 per 1000 patients in 2010. Mortality for patients with ESRD decreased nonsignificantly from period 1 to 2 (RR 0.72; P = 0.52), but significantly from period 1 to 3 (RR 0.24; P = 0.006), whereas for patients without ESRD mortality decreased significantly for all comparisons. ESRD was associated with a high overall mortality (RR 9.9; 95% CI 6.3-14.5; P < 0.0001).
Conclusion
As a result of longer survival, the prevalence of ESRD is increasing but remains associated with a high mortality. The incidence of ESRD declined in Black but not in Caucasian patients. IDU and HCV were identified as additional risk factors for the development of ESRD.
Introduction
The prevention and treatment of cardiovascular, liver and kidney diseases have increasingly been the focus of attention in attempts to reduce the morbidity and mortality of HIV-infected individuals [1]. Renal disease of any stage is a common complication in HIV-infected patients, affecting up to 30% of patients, and is associated with increased morbidity and mortality [2-4]. Once established, end-stage renal disease (ESRD) and chronic renal replacement therapy (RRT) substantially increase the risk of death and cardiovascular events in the general and HIV-infected populations [5-7]. The causes of kidney disease in HIV-infected patients are heterogeneous and include diseases related to HIV [HIV-associated nephropathy (HIVAN), immune complex glomerulonephritis and thrombotic microangiopathy], chronic hepatitis virus coinfection and factors not directly related to HIV (drug toxicity, age, diabetes mellitus and hypertension). Traditional risk factors for ESRD are likely to increase over time with aging of the HIV-infected population [8-10].
In the USA, HIV-associated ESRD has become epidemic among Black patients [11, 12]. Black individuals are more prone to kidney disease than any other ethnic group in the general [13, 14] and HIV-infected populations, possibly as a consequence of predisposing genetic polymorphisms [15]. The risk of ESRD in Black HIV-infected individuals has been reported to be three- to six-fold higher than in Caucasian HIV-infected patients [7, 16, 17], reflecting the susceptibility of Black patients to develop HIVAN. The introduction of highly active antiretroviral therapy (HAART) has reduced the incidence of HIVAN and ESRD among Black patients [18-20], but the prevalence of ESRD is projected to further increase [11, 12]. Earlier studies, mainly from the USA, concluded that, almost exclusively in Black patients, HIVAN is one of the major causes of ESRD [6, 21, 22], but histological confirmation of HIVAN is infrequent and the diagnosis is usually made clinically. More recent studies have reported an increasing relevance of traditional risk factors as well as chronic hepatitis C virus (HCV) coinfection in the development of ESRD in HIV-infected patients [7, 23].
The epidemiology of ESRD outside the USA has not been extensively studied [24-27] but could provide new insights, as most US cohorts consist of a high proportion of Black patients with coeval injecting drug use (IVDU). Conversely, European cohorts consist of mostly Caucasian HIV-infected patients with various proportions of IDU and far fewer Black patients with almost no IDU [19, 26, 28].
Thus, the aim of our study was to evaluate the incidence, prevalence and outcome of patients with ESRD since the initiation of a chronic renal replacement therapy (RRT) programme in a large, single German HIV-infected cohort.
Methods
Data for all HIV-infected patients treated from January 1989 to December 2010 in the Frankfurt HIV Cohort (FHC) were evaluated for this study. Patients are routinely followed every 3 − 4 months with the use of paper charts and a standardized electronic database. ESRD was defined as initiation of chronic RRT for more than 3 months. Demographic, clinical and laboratory parameters of all patients included in the FHC and those with ESRD were compared. Chronic hepatitis B virus (HBV) coinfection was defined as a detectable HBV surface (HBs) antigen persisting for at least 6 months; HCV coinfection was diagnosed when patients tested positive for HCV antibodies. Three time periods, 1989-1996 (pre-HAART), 1997-2003 (early HAART) and 2004-2010 (late HAART), were defined to describe changes over time.
Continuous variables were expressed as mean ± standard deviation (SD) or as proportions, as appropriate. Continuous and categorical variables were compared for univariate analysis between groups using the t-test or Wilcoxon test and the χ2 or Fisher exact test, respectively. All P values reported are two-sided and confidence intervals (CIs) are 95% intervals. Statistical significance was defined as P ≤ 0.05. The association between the presence of ESRD and demographic and clinical variables was estimated by Poisson regression analysis [relative risk (RR) and confidence interval (CI)]. Kaplan−Meier estimates were used to analyse the survival of ESRD patients.
Results
Study population
Between 1 January 1989 and 31 December 2010, a total of 9198 patients were followed in the cohort, with a cumulative observation time of 68 084 patient-years (PY). The majority were male (78.2%), Caucasian (88.9%) and men who have sex with men (MSM) (49.9%). In the entire cohort, the percentage of Black patients, those with heterosexual acquisition of HIV infection, and those with HBV and HCV coinfection increased from the pre-HAART (1989-1996) to the late HAART (2004-2010) time period (P < 0.01 for all comparisons), whereas such a change could not be detected in the group of patients with ESRD. The percentage of male patients, the percentage of patients with IDU, the percentage of those with previous AIDS, and mortality significantly decreased in the entire cohort (P < 0.01 for all comparisons) (Table 1).
Prevalence and incidence of ESRD
Over the whole observation period, between 1989 and 2010, 39 patients in our cohort had ESRD, with a mean prevalence of 0.12%. ESRD was more prevalent in Black patients compared with Caucasians (mean prevalence 0.6 vs. 0.09%, respectively). The prevalence of ESRD continuously increased over time from 0.08% in the first defined time period to 0.19% in the third time period. While prevalence increased in Caucasian patients (from 0.05 to 0.17%), in Black patients it was highest in 1995, at 2.6%, and decreased thereafter to 0.38% in the third time period.
Incidence was calculated for 35 of the 39 patients, because one patient had ESRD before he became HIV-infected and three patients were referred to our centre for the initiation of RRT and thus entered the cohort after they had developed ESRD. The overall incidence of ESRD was 51.5 per 100 000 PY of follow-up (PYFU) (95% CI 35.9-71.6) and was significantly higher for Black compared with Caucasian patients. The incidence of ESRD in Black patients decreased substantially over the three defined time periods, from 788.8 to 130.5 and 164.1 per 100 000 PYFU, respectively, but increased in Caucasian patients, from 29.8 to 41.0 and 43.4 per 100 000 PYFU, respectively.
Risk factors for ESRD and mortality
Compared with the entire cohort, patients with ESRD were also mostly male (82.1 vs. 78.2%; P = 0.69) but were more likely to be Black (25.6 vs. 7.4%; P < 0.0001), IDUs (33.3 vs. 16.1%; P = 0.0067) and HCV-coinfected (38.5 vs. 11.0%; P = 0.037). HBV coinfection (12.8 vs. 4.2%; P = 0.46) and previous AIDS (43.6 vs. 30.1%; P = 0.095) were also more frequent in patients with ESRD but the difference was not stastically significant (Table 2). In the univariate analysis, risk factors for the development of ESRD were Black ethnicity (RR 5.1; 95% CI 2.3-10.3; P < 0.0001), IDU (RR 2.4; 95% CI 1.1-4.7; P < 0.02) and HCV coinfection (RR 1.7; 95% CI 1.1-4.2; P < 0.03). In the multivariate regression analysis, Black ethnicity (RR 6.7; 95% CI 2.8-14.8; P < 0.00001) and IDU (RR 5.0; 95% CI 1.4-16.9; P = 0.014) but not HCV coinfection (RR 0.84; 95% CI 0.23-2.9; P = 0.79) remained significantly associated with the development of ESRD (Table 3). No association was found between age, gender, nadir CD4 lymphocyte count or previous AIDS-defining disease and ESRD. In separate analysis of Caucasian and Black patients, we found that prior AIDS was not a risk factor for ESRD in either Black (RR 2.6; 95% CI 0.65-8.96; P = 0.15) or Caucasian patients (RR 1.6; 95% CI 0.61-3.6; P = 0.31).
ESRD was associated with an overall high risk of mortality (RR 9.9; 95% CI 6.3-14.5; P < 0.0001). The mortality of patients with ESRD decreased over the three defined time periods, from 5714.7 per 10 000 PY in the pre-HAART (period 1) to 4100.5 per 10 000 PY in the early HAART (period 2) and finally to 1369.4 per 10 000 PY in the late HAART period (3). The corresponding RR of death for patients with ESRD decreased insignificantly from period 1 to 2 (RR 0.72; P = 0.52), but significantly from period 1 to 3 (RR 0.24; P = 0.006), whereas in patients without ESRD, mortality decreased significantly for both comparisons (period 1 vs. 2: RR 0.17; P < 000001, and period 1 vs. 3: RR 0.10; P < 0.000001). Figure 1 shows the Kaplan−Meier survival plot for patients with ESRD for the three defined time periods (P = 0.069 for the log rank test). The median survival time was 15.8, 20.7 and 28.8 months in the three time periods, respectively.
Spectrum of renal disease and renal replacement therapy
In 34 of our 39 patients developing ESRD, the underlying cause of renal disease could be established. Diagnosis was based on renal biopsy in 31 cases and was made by other diagnostic means in three cases (e.g. by ultrasound findings in two patients with a known family history of autosomal dominant polycystc kidney disease). The spectrum of renal disease observed was highly heterogeneous, with diabetic nephropathy being the most frequent cause of ESRD (nine of 34 patients; 26.5%), whereas HIVAN was rather uncommon (four of 34 patients; 11.8%). Haemodialysis was the treatment of choice for RRT in all patients. The mean time from the first positive HIV antibody test to initiation of RRT was 81.2 ± 74.5 months, and RRT was initiated prior to or within 4 weeks of HIV diagnosis in eight patients (20.5%). The mean observation time on RRT was 32.1 ± 30.2 months. Two patients moved abroad and were lost to follow-up. Comorbidities were frequent: one-third of the patients with ESRD had cardiovascular disease and one-fourth had malignancies. Causes of death were mainly attributable to infections (30.4%), followed by malignancies (21.7%) and cardiovascular disease (17.4%). Mean age at death was 45.2 ± 12.2 years. Demographic data and clinical characteristics are shown in Table 4.
|
|
| |
| |
|
|
|