| |
Differential Efficacy of ABX464 and its Primary Metabolite ABX464-NGlc on
HIV Replication: Implications for Treatment Strategies to Eliminate Viral Reservoirs
|
| |
| |
Reported by Jules Levin
HIV Glasgow Oct 23-26 2016
----------------
"Phase IIa trial in HIV/AIDS patients with drug candidate
ABX464"....treatment/cure....http://www.natap.org/2016/HIV/060116_02.htm
"Study ABX464-004, the second Phase IIa trial conducted with ABX464, is designed to demonstrate the long-lasting effect of ABX464, which has been observed in preclinical studies. The study will enroll twenty-eight patients whose HIV infection is already fully controlled by boosted Darunavir. ABX464 will be administered to 21 of these patients in combination with their current drug regimen, while the remaining 7 patients will be given placebo in combination with their current therapy. After 28 days, all treatment will be discontinued and the study will then measure the time elapsed until the HIV virus reappears in the blood of the ABX464-treated patients and the control group. The efficacy endpoint of the study is the time to rebound of the viral load. This rebound will originate from the HIV reservoirs, which are not affected by current combination antiretroviral treatment. Preliminary results of this study are expected in Q4 2016.
An increase in the time to viral load rebound would constitute the first successful attempt at providing a functional cure for the HIV infection. Large-scale pivotal clinical studies of ABX464 will be required to confirm this hypothesis. These studies could begin by early
2017."http://www.businesswire.com/news/home/20160530005192/en/Ongoing-Clinical-Development-ABX464-ABIVAX-Launches-ABX464-004
http://www.abivax.com/en/investors/press-releases/20-treatment-of-the-first-hiv-positive-patient-in-abivax-s-phase-iia-clinical-trial-with-abx464.html Pending confirmation in clinical trials, this unique mode of action and preclinical data to-date suggest that ABX464 could:
⋅Induce long term control of the viral load
⋅Not induce HIV mutants that are resistant to treatment
⋅Be less frequently administered over a shorter period than standard treatments;
providing the potential to reduce healthcare costs and offer broader access to treatment
---------------------------
D. Scherrer 1*, J.-M. Steens1, P. Gineste1, N. Campos1, A. Garcel1, E. Schlaefper 2, R. Speck 2, J. Tazi 3, H.J. Ehrlich1
1ABIVAX, Paris and Montpellier, France; 2 University of Zurich Hospital, Zurich, Switzerland; 3 University of Montpellier, CNRS UMR 5535, Montpellier, France; *Presenting author.
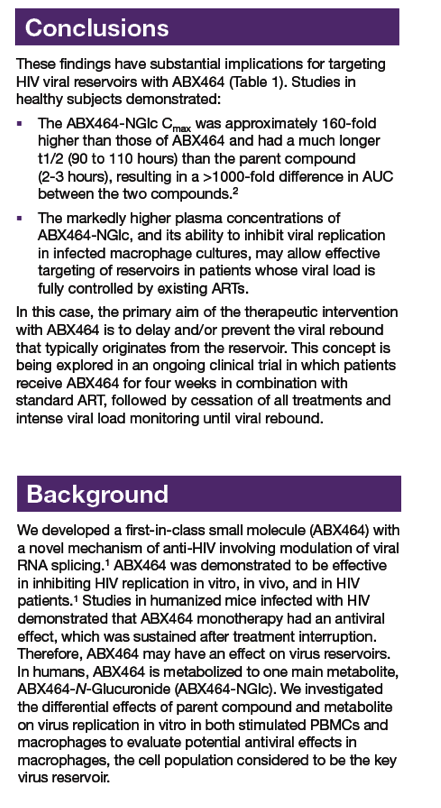
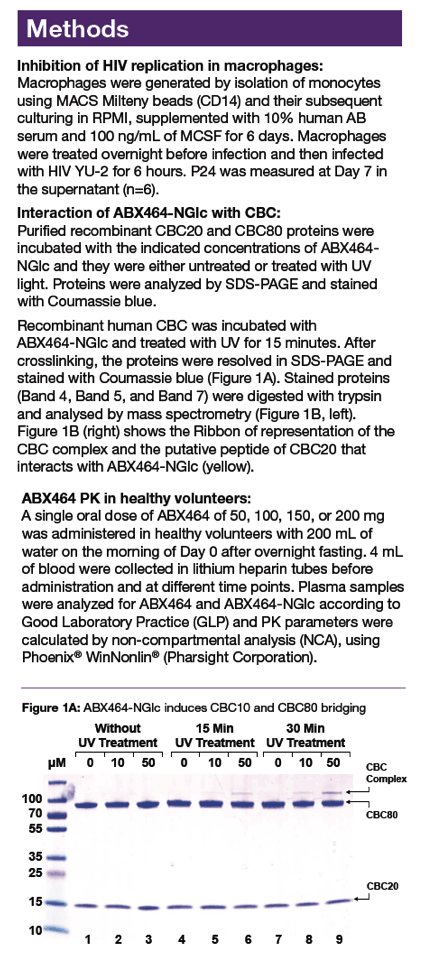
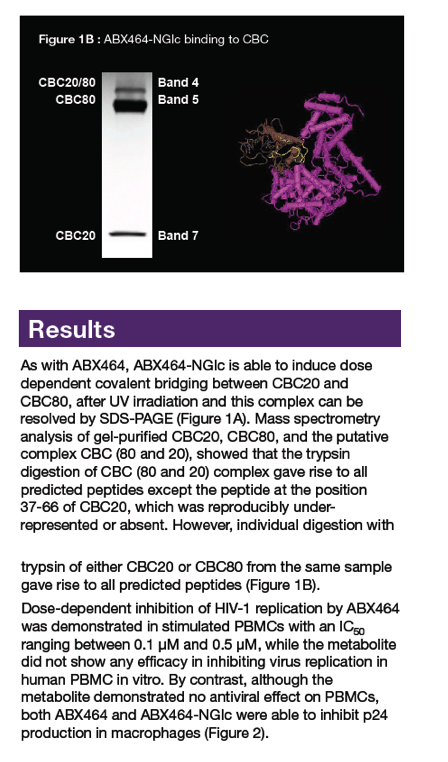
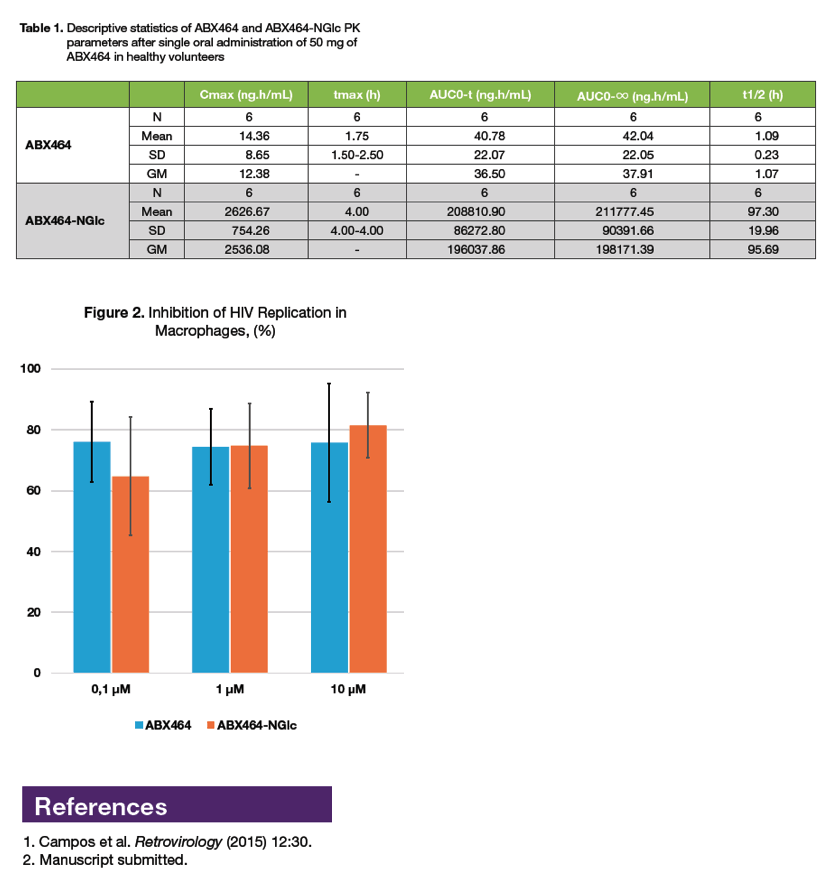
|
|
| |
| |
|
|
|