 |
 |
 |
| |
The impact of Universal Test and Treat on HIV incidence
in a rural South African population ....Poor Linkage to Care Undermines Test-and-Treat in South African Trial....
|
| |
| |
21st International AIDS Conference (AIDS 2016), July 18-22, 2016, Durban, South Africa
Mark Mascolini
A test-and-treat strategy did not lower HIV incidence in a 28,000-person cluster-randomized trial in rural KwaZulu-Natal province, South Africa [1]. But 95% of participants who started antiretroviral therapy (ART) in the test-and-treat arm and the standard-of-care arm reached an undetectable viral load. In a press release the French ANRS team that conducted the trial concluded that "entry into the healthcare system of people diagnosed as HIV seropositive is too infrequent and slow to reduce HIV transmission in [this] population."
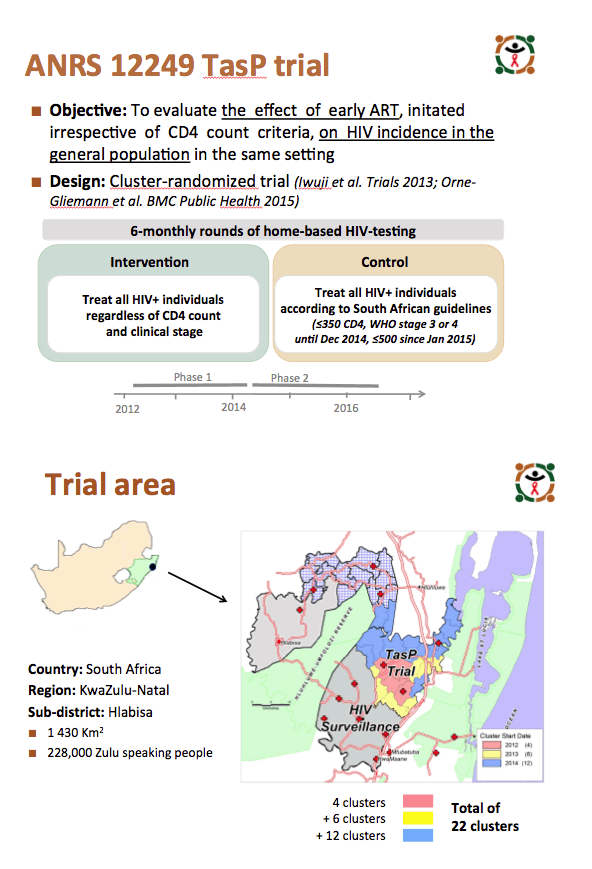
ANRS 12249 TasP is the first of five trials to provide data on acceptance of a universal test-and-treat strategy in a high-risk population and on whether that strategy can limit new HIV infections by diagnosing a high proportion of HIV-positive people and linking them to effective antiretroviral therapy.
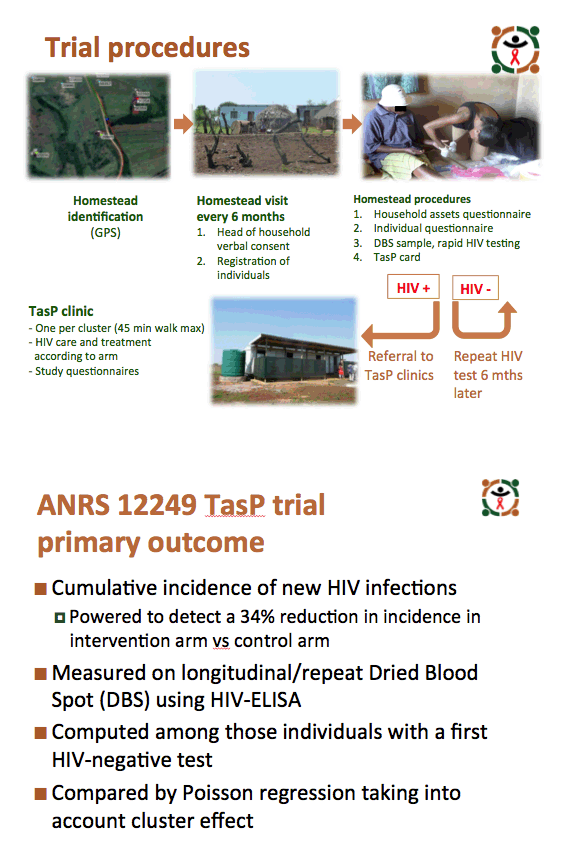
The trial took place in 22 geographic zones of KwaZulu-Natal's Hlabisa subdistrict, which has an HIV prevalence of 29%, the highest in South Africa. Health workers offered rapid HIV testing in home-based survey rounds every 6 months to all residents 16 years old or older. Researchers randomly distributed the 22 zones to the intervention arm (ART regardless of CD4 count) or to the control arm (ART according to national guidelines). People who tested positive got referred to cluster-based trial clinics for HIV care. People in the control arm could also go to Department of Health clinics.
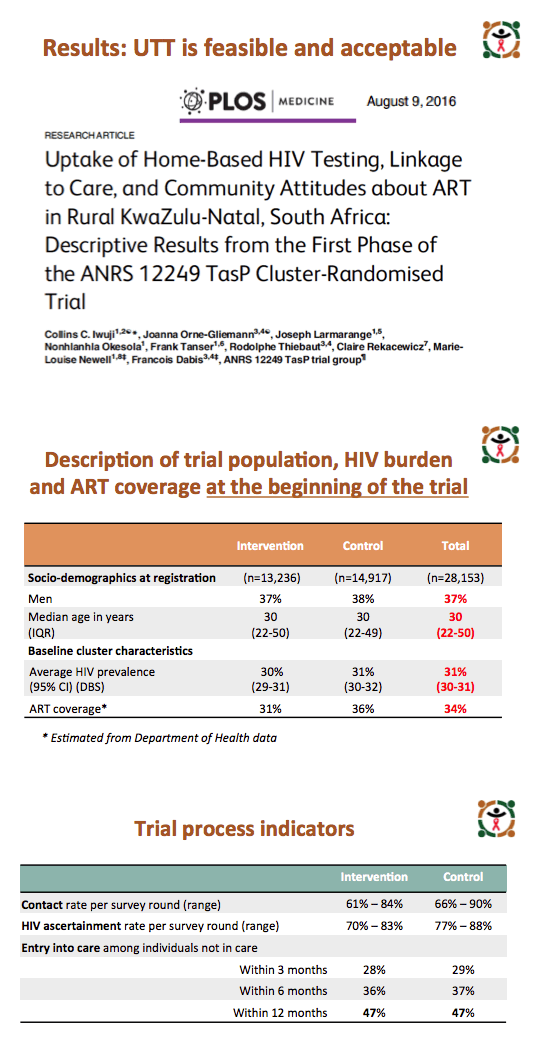
From March 2012 through April 2016, the ANRS team registered 13,239 people in the intervention arm and 14,917 in the control arm. In the intervention and control arms, proportions of men were 37% and 38%, and median age was 30 years in both arms. HIV prevalence stood at 30% in the intervention group and 31% in the control group, and respective proportions already taking ART were 31% and 36%.
HIV ascertainment rate per survey round ranged from 70% to 83% in the intervention arm and from 77% to 88% in the control arm. But among people who tested positive, entry to care proved slow and suboptimal, at 28% and 29% in intervention and control arms within 3 months of HIV diagnosis, at 36% and 37% within 6 months, and at 47% in both arms within 12 months.
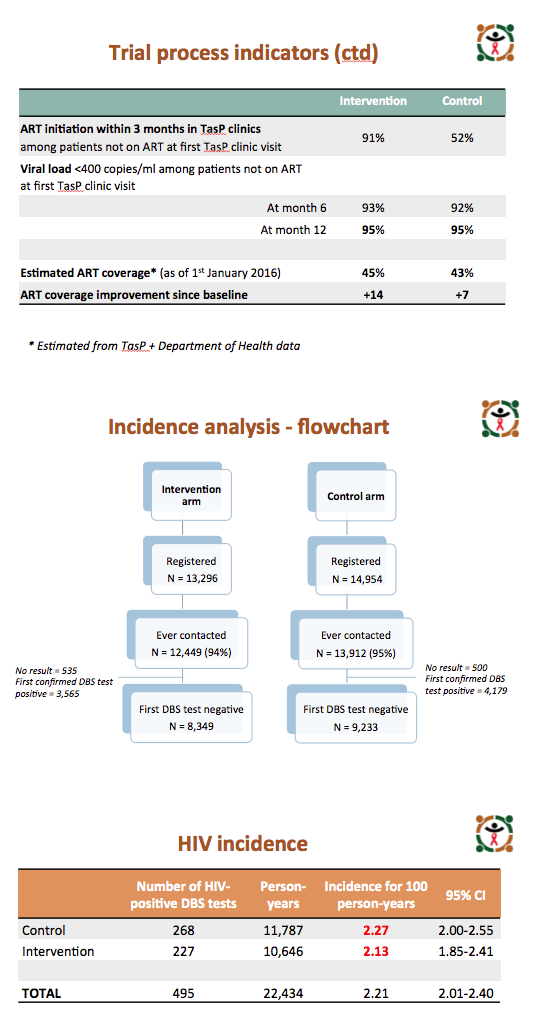
Among people not already taking ART at their first clinic visit, 91% in the intervention arm and 52% in the control arm began ART within 3 months of their first visit. Among people who began ART during the trial, more than 90% in both arms had a viral load below 400 copies 6 months after starting therapy, and 95% in both arms had a sub-400 viral load by month 12. But estimated ART coverage among HIV-positive people reached only 45% in the intervention arm and 43% in the control arm by January 1, 2016.
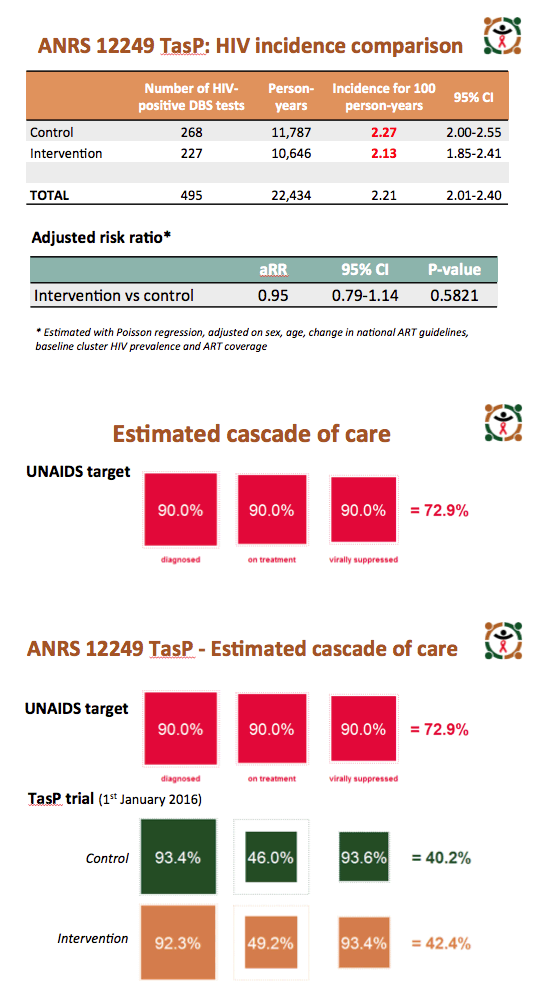
Among people registered and contacted in the trial, 8349 in the intervention arm and 9233 in the control arm tested negative on their first HIV test. On subsequent tests, 227 initially negative people in the intervention group and 268 in the control group tested positive to yield an HIV incidence of 2.13 (95% confidence interval [CI] 1.85 to 2.41) in the intervention arm and 2.27 (95% CI 2.00 to 2.55) in the control arm. Poisson regression analysis adjusted for age, sex, change in national ART guidelines, baseline cluster HIV prevalence, and ART coverage determined that the intervention did not yield a significant advantage in preventing new HIV infections (adjusted risk ratio 0.95, 95% CI 0.79 to 1.14, P = 0.58).
The World Health Organization set 2020 targets of 90% of HIV-positive people diagnosed, 90% of those diagnosed on treatment, and 90% of those on treatment achieving viral suppression (72.9% of all HIV-positive people with viral suppression). Respective proportions in the intervention arm were 92.3%, 49.2%, 93.4% (42.4%) and in the control arm 93.4%, 46.0%, 93.6% (40.2%).
The researchers surmised that prevailing models of care and community attitudes and stigma could account for the poor linkage that thwarted the test-and-treat strategy in this trial. Principal investigators Francois Dabis and Deenan Pillay proposed that "innovative research on the healthcare system and communities must be conducted to reach the targets of the universal test and treat strategy, if we hope one day to control the epidemic."
Reference
1. Iwuji C, Orne-Gliemann J, Balestre E, et al. The impact of universal test and treat on HIV incidence in a rural South African population: ANRS 12249 TasP trial, 2012-2016. 21st International AIDS Conference (AIDS 2016). July 18-22, 2016. Durban, South Africa. Abstract FRAC0105LB.


|
| |
|
 |
 |
|
|