 |
 |
 |
| |
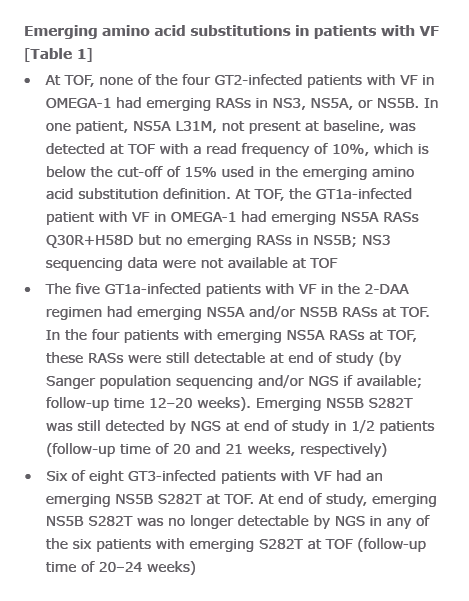
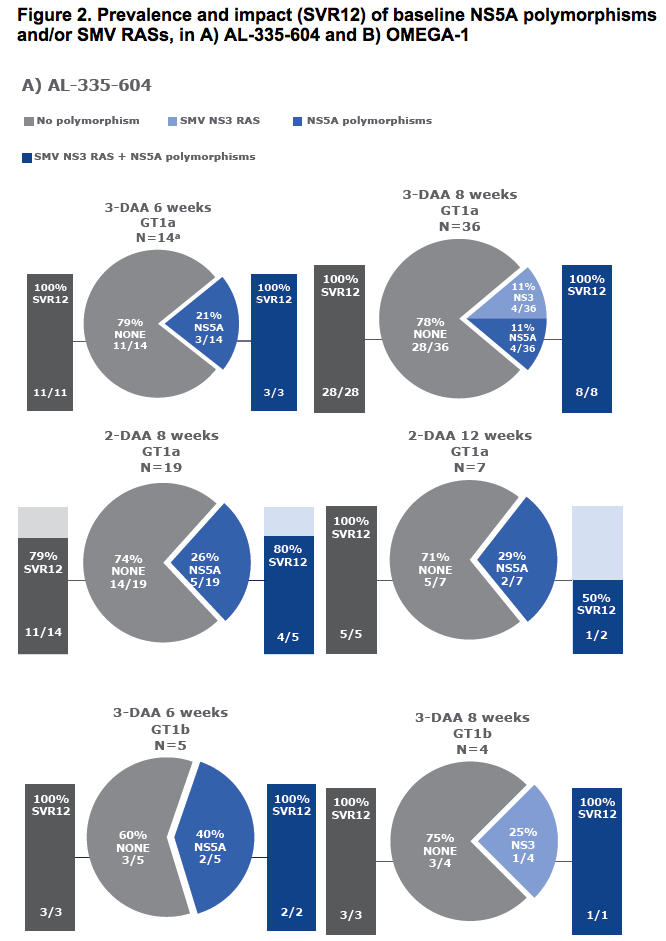
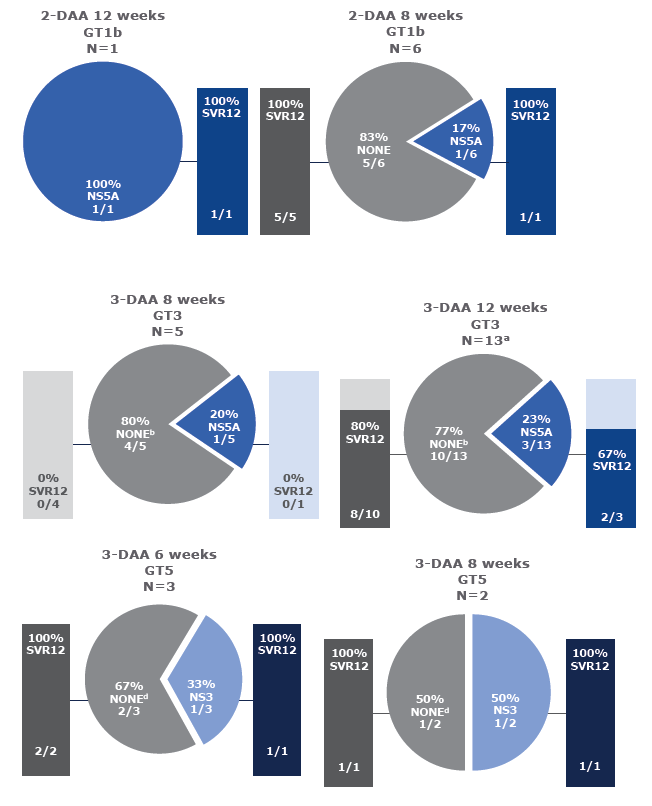
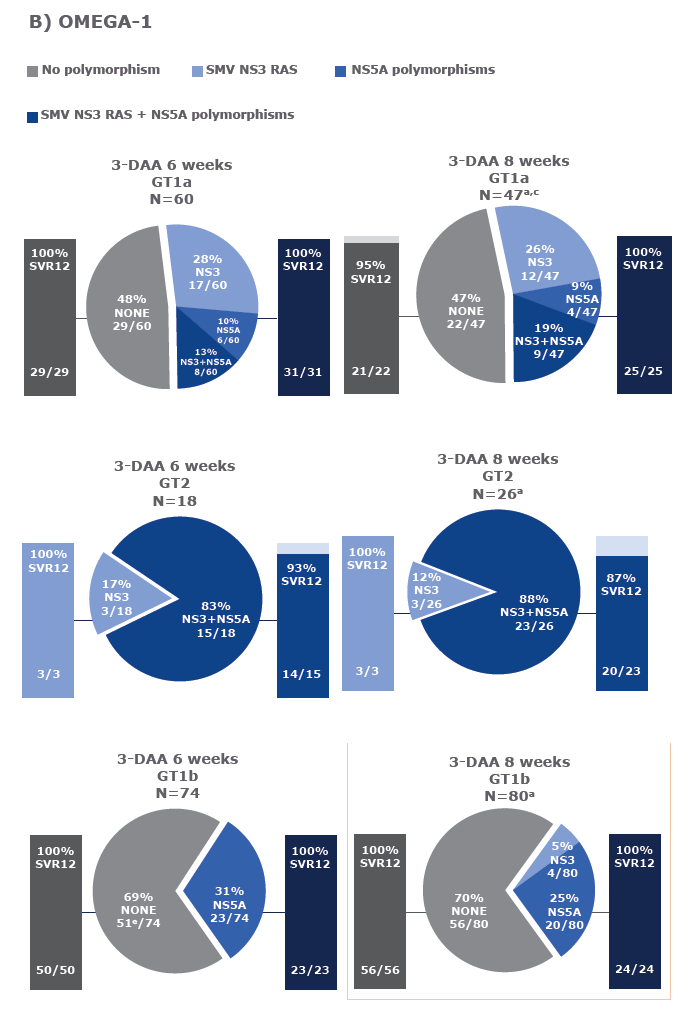
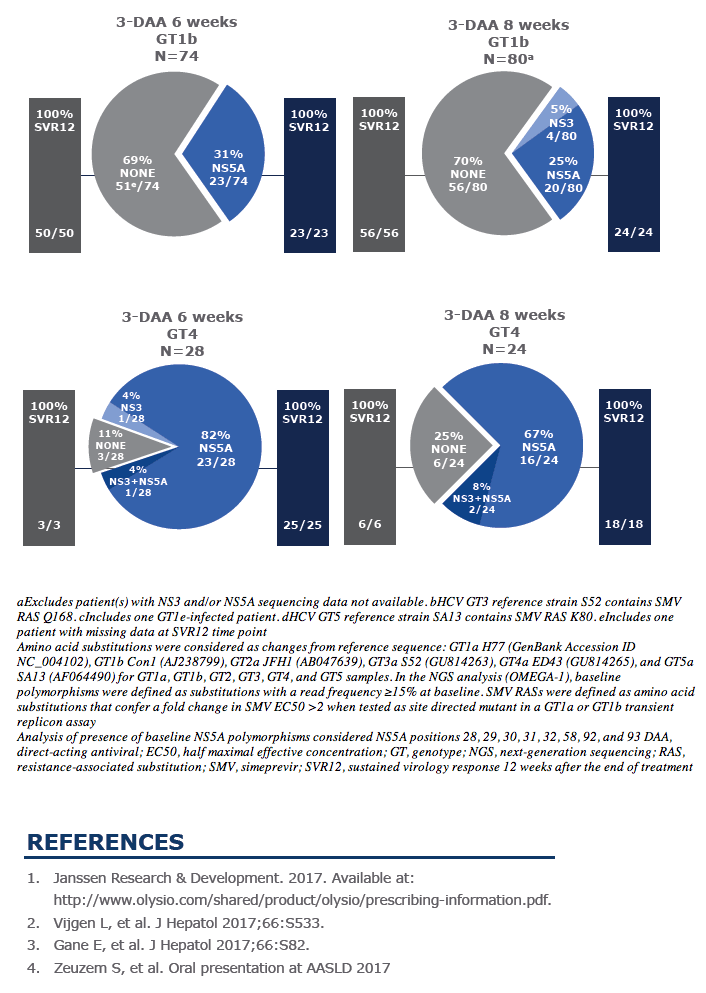
Virology analyses from HCV-infected patients treated with the combination of AL-335 and odalasvir, with and without simeprevir, in Phase II studies
|
| |
| |
Reported by Jules Levin
AASLD 2017 Wash Dc Oct 20-24
AASLD: Evaluation of the efficacy and tolerability of JNJ-4178 (AL-335, odalasvir, and simeprevir) in hepatitis C virus-infected patients without cirrhosis: The Phase IIb OMEGA-1 study - (10/23/17)
L. Vijgen,1 B. Fevery,1 M. Biermer,1 M. McClure,2 R. Sinha,3 W. Willems,1 C. Corbett,1 C. Liu,3 I. Augustyns,1 L. Chen,3 E. J. Gane,4 S. Zeuzem,5 C. Moreno,6
P. Buggisch,7 E. Tam,8 S. De Meyer1
1Janssen Research & Development, Janssen Pharmaceutica NV, Beerse, Belgium; 2Alios BioPharma Inc., part of the Janssen Pharmaceutical Companies, South San Francisco, CA, USA; 3Janssen Research & Development, LLC, Titusville, NJ, United States; 4New Zealand Liver Transplant Unit, University of Auckland, Auckland, New Zealand; 5J.W. Goethe University Hospital, Frankfurt am Main, Germany; 6CUB Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium; 7Institute for Interdisciplinary Medicine, Hamburg, Germany; 8LAIR Centre, Vancouver, B.C., Canada

|
| |
|
 |
 |
|
|