 |
 |
 |
| |
A Phase 2 Open-Label Trial of Antibody UB-421 Monotherapy as a Substitute for HAART
|
| |
| |
Reported by jules Levin
CROI 2017 Feb 14-16 Seattle, WA
Chang-Yi Wang, PhD1, Wing-Wai Wong, MD2, Hung-Chin Tsai, MD, PhD3, Yen-Hsu Chen, MD, PhD4, Mei-June Liao, PhD5, Shugene Lynn, PhD6
1 United Biomedical Inc. (UBI), NY, USA; 2Taipei Veterans General Hospital, Taiwan; 3 Kaohsiung Veterans General Hospital, Taiwan;
4 Kaohsiung Medical University Hospital, Taiwan; 5 United Biopharma Inc., Taiwan; 6 UBI Asia, Taiwan
Abstract Body:
The antiviral activity of UB-421, a monoclonal antibody that binds to CD4 receptor to block HIV-1 entry, was demonstrated in a prior Phase 2a trial of 29 antiretroviral-naïve HIV-1(+) adults in Taiwan (Trial No. NCT01668043); the average (and SD) of maximum viral load (VL) reduction was 2.27 (0.6) and 2.45 (0.46) log10 copies/mL after 8-week monotherapy on 10mg/Kg/weekly or 25mg/Kg/biweekly, respectively.
Efficacy and safety of UB-421 monotherapy was examined in this Phase 2 trial among HAART-stabilized HIV-1(+) Asian adults whose VLs had been recorded as undetectable (< 50 copies/mL) at least twice in the past year (NCT02369146); 14 and 15 such adults interrupted HAART to receive 8 doses of UB-421 infusions on 10mg/Kg/weekly (Arm 1) or 25mg/Kg/biweekly (Arm 2) regimens, respectively, with prompt return to HAART if viral rebound (VR, > 400 copies/mL at 2 consecutive visits). Efficacy was assessed as the percentage of and the time to VR; changes in laboratory parameters were compared to baseline.
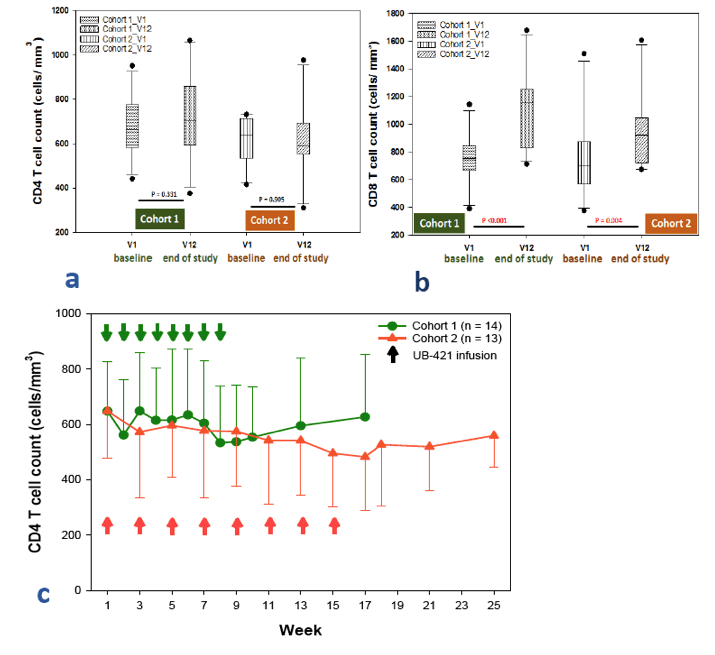
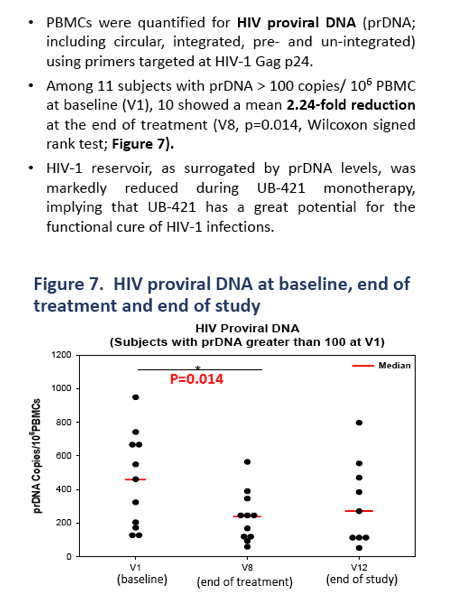
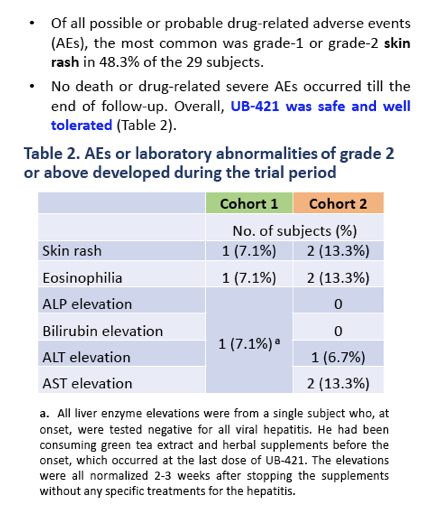
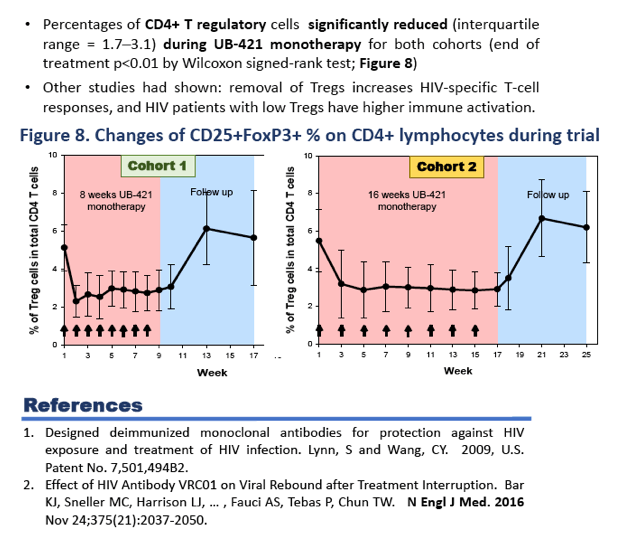
Twenty-seven of 29 enrolled subjects completed all 8 doses of UB-421 with no VR during the monotherapy period, which was 8-week for Arm 1 and 16-week for Arm 2. Two subjects in Arm 2 did not complete (1 lost to follow-up, 1 withdrew due to skin rash), but had undetectable VL for all trial visits. At the end of treatment (EOT), 22 subjects resumed HAART and were monitored for 8 weeks with continuous viral suppression. Five subjects (3 in Arm 1 and 2 in Arm 2) refused to resume the scheduled HAART, and viral rebound was detected 35-62 days after the last UB-421 dose; all 5 re-started HAART right after rebound, and their VL were monitored until undetectable. At the end of study for both arms, CD4+ T cell counts remained stable (p>0.17 Wilcoxon signed-rank (WSR) test), while CD8+ T cell counts increased (p<0.05). The 27 completers exhibited significant reductions (interquartile =1.7% - 3.1%) of CD4+ T regulatory cell percentage at EOT (p<0.001 WSR test). In 11 subjects with proviral DNA > 100 copies/106 PBMC at baseline, 10 showed a mean 2.24-fold reduction at EOT. The most common drug-related (possible or probable) adverse event (AE) was mild to moderate skin rash (48.3% of 29 subjects), and no death or drug-related SAE occurred.
No viral rebound occurred during the UB-421 monotherapy period; the regimen was safe and well tolerated. UB-421 warrants further evaluation for an indication as monotherapy for HAART substitution in virally suppressed HIV-1 adults.

|
| |
|
 |
 |
|
|