 |
 |
 |
| |
Neuropsychiatric Adverse Events Associated With Integrase Strand Transfer Inhibitors
|
| |
| |
Reported by Jules Levin
CROI 2017 Feb 14-16 Seattle, WA
Prabha Viswanathan, Elande Baro, Guoxing Soon, Adam Sherwat, Kimberly Struble Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, MD USA

Meta-Analysis
⋅The primary analysis is based on a meta-analysis with fixed effects. This model assumes a common effect size shared by all the studies (no heterogeneity across the studies). The estimate of the overall risk difference was computed as a weighted average of the study specific risk differences. Studies with smaller variance were given more weight.
⋅As a sensitivity analysis, a meta-analysis with random effects (DerSimonian-Laird method) was performed. The model estimates were adjusted to incorporate a measure of the variability across studies.
Supportive Analysis
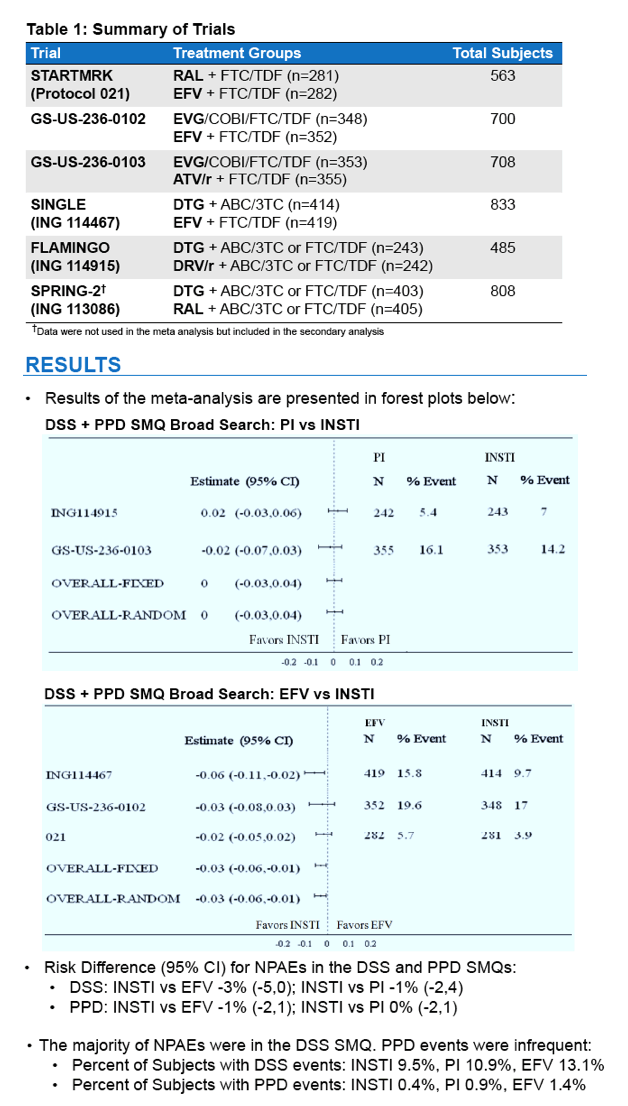
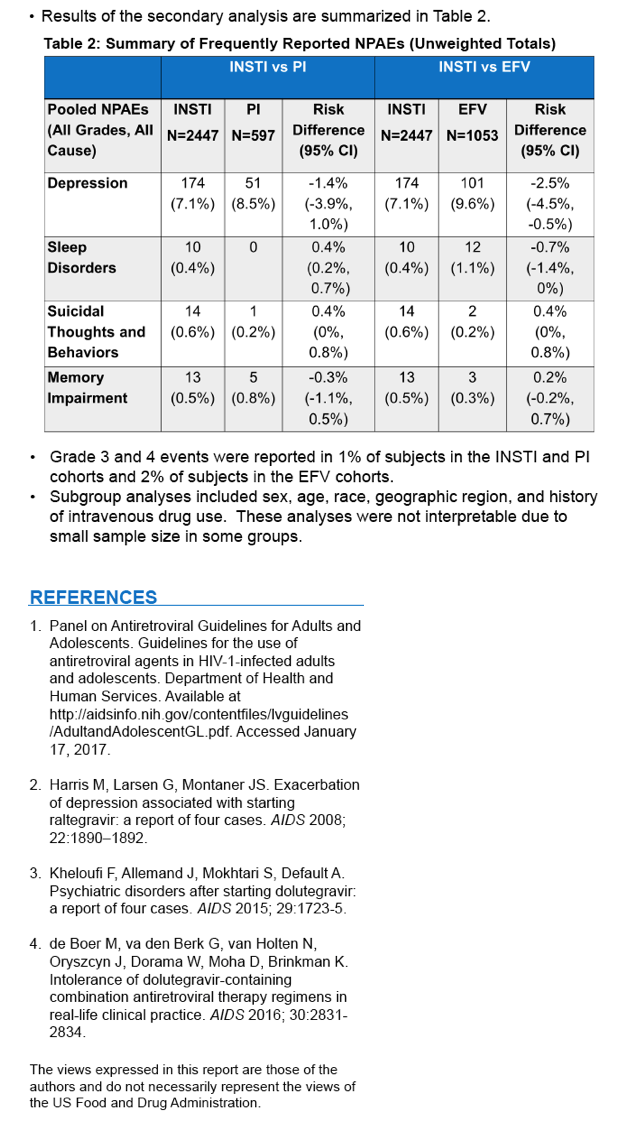
⋅FDA conducted a supportive analysis comparing the proportion of subjects with NPAEs among the three treatment groups: INSTI, EFV, and PI.
⋅This analysis was performed with data from the 5 clinical trials included in the meta-analysis, plus SPRING-2.
|
| |
|
 |
 |
|
|