 |
 |
 |
| |
Predictors of Stopping Dolutegravir or Elvitegravir in Large Netherlands Group - ATHENA Cohort
|
| |
| |
16th European AIDS Conference, October 25-27, 2017. Milan
Mark Mascolini
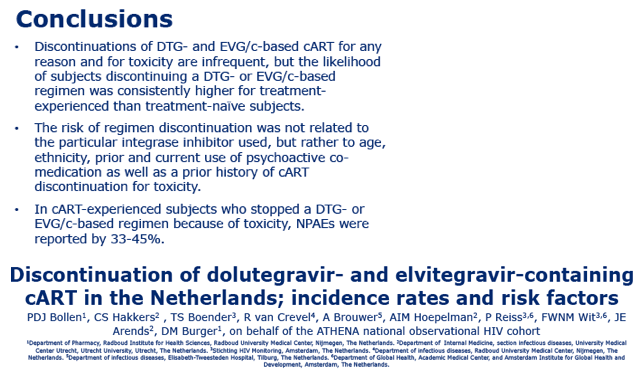
Stopping treatment with dolutegravir (DTG) or elvitegravir (EVG), the newer integrase inhibitors, proved rare in a 3416-person analysis in the Netherlands [1]. Previous antiretroviral discontinuation, older age, and ever using psychoactive drugs predicted stopping one of these drugs.
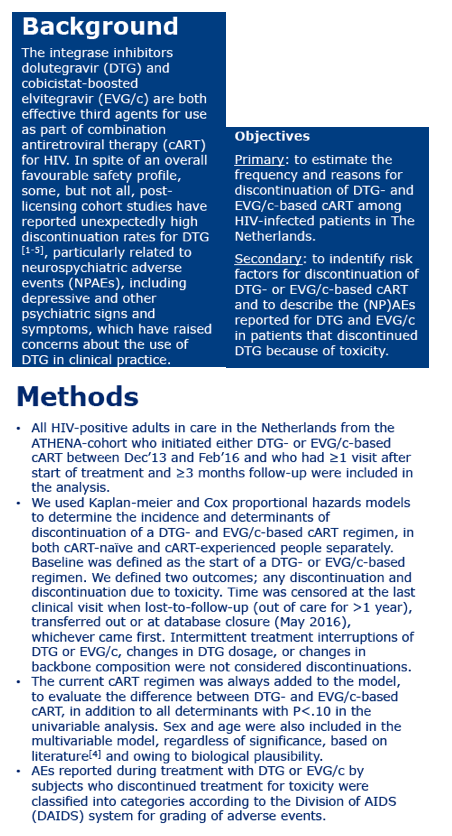
Because of their potency and relative safety, integrase inhibitors see increasing use in first-line and rescue regimens. But some postapproval studies found unexpectedly high rates of stopping DTG or EVG, often because of neuropsychiatric adverse events, including depressive symptoms. Researchers at Nijmegen's Radboud University and colleagues at other centers undertook this study to estimate frequency of stopping either of these integrase inhibitors, and to identify reasons for quitting.
The analysis involved all HIV-positive adults in the Netherlands ATHENA cohort who began DTG or EVG between December 2013 and February 2016 and had at least 1 visit and 3 months of follow-up after starting the integrase inhibitor. The researchers used Kaplan-Meier analysis to chart incidence of DTG or EVG discontinuation and Cox proportional hazards models to determine reasons for stopping.
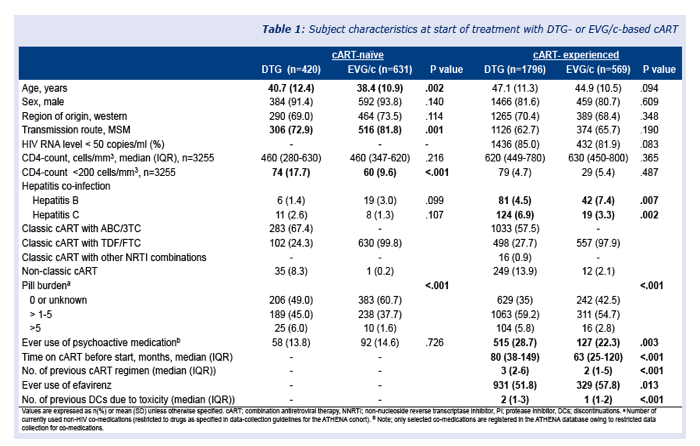
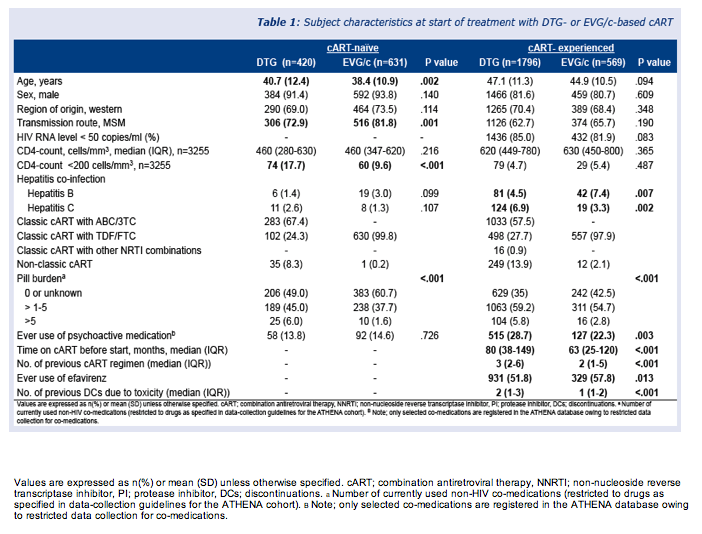
The study included 3416 people starting one of the two integrase inhibitors, 1051 of them (31%) in a first-line regimen. Median follow-up reached 8.4 months in antiretroviral-naive people and 9.0 months in treatment-experienced people. Median ages of DTG takers were 40.7 among the naive and 47.1 among the experienced. Respective ages of EVG takers were 38.4 and 44.9. In the antiretroviral-naive group, 13.8% starting DTG and 14.6% starting EVG ever used psychoactive drugs. Respective proportions in the experienced group were 28.7% and 22.3% (P = 0.003).
Significantly higher proportions of antiretroviral-experienced than naive people stopped either DTG or EVG for any reason or for toxicity (P < 0.0001). Discontinuation rates did not differ significantly between the two integrase inhibitors. Through 12 months of follow-up, about 15% of experienced people stopped either drug for any reason, and about 10% of previously untreated people stopped either drug for any reason.
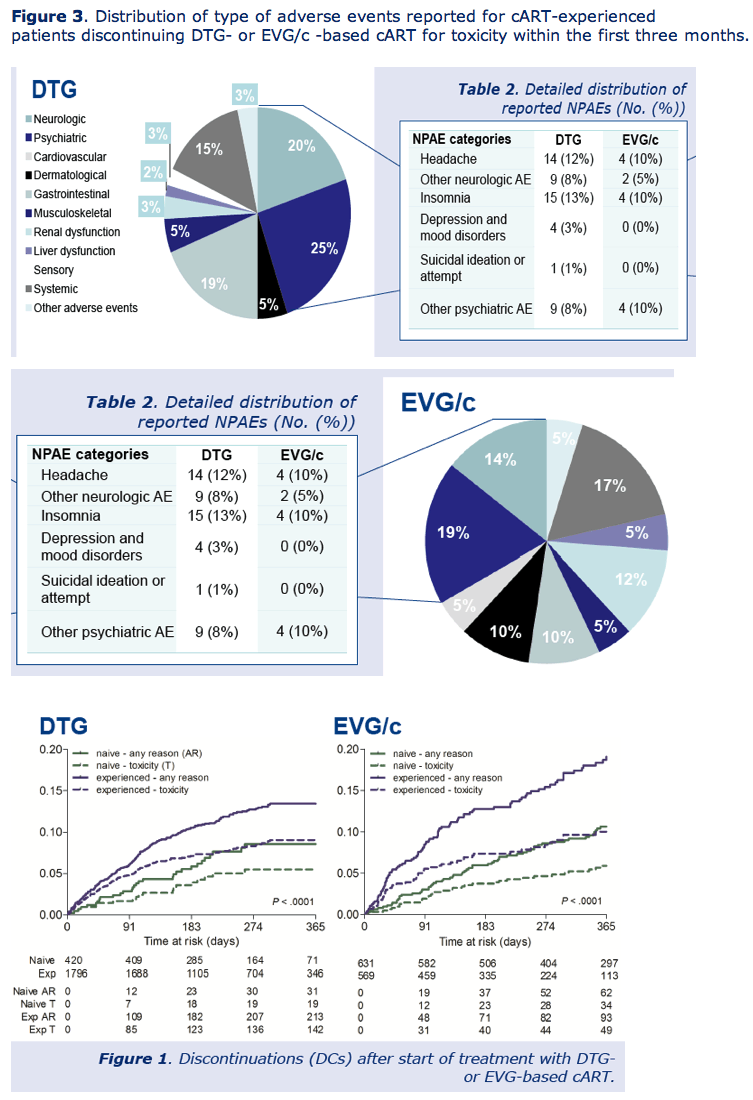
Toxicity accounted for most discontinuations in both naive people (5.6%) and experienced people (8.2%) with both drugs, independently of which agent they took. Within 3 months of starting their integrase inhibitor, 4.7% of treatment-experienced people stopped DTG and 5.4% stopped EVG for toxicity. In this antiretroviral-experienced group, 2.6% stopped DTG and 2.3% stopped EVG because of toxicity and also reported neuropsychiatric adverse events.
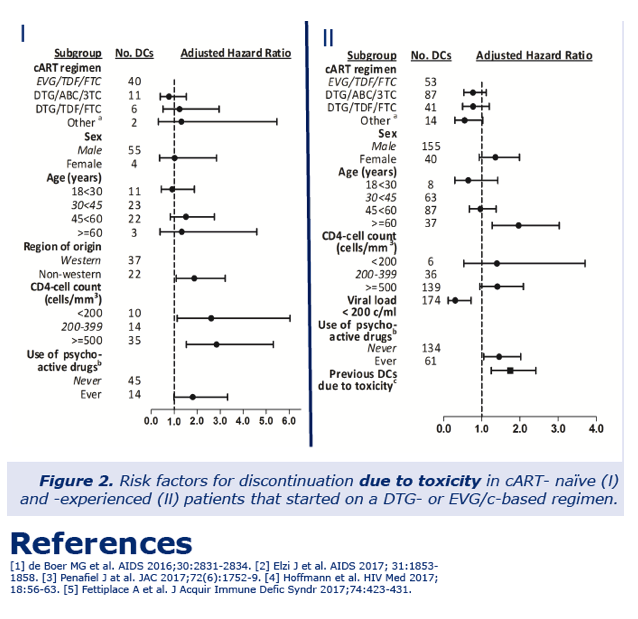
Multivariable analysis found no significant difference between DTG and EVG in discontinuation for any reason or for toxicity. This analysis identified four factors independently associated with stopping for toxicity among antiretroviral-naive people at the following hazard ratios (HR) (and 95% confidence intervals):
-- Non-Western origin: HR 1.87 (1.09 to 3.22), P = 0.023
-- Age 60 or older: HR 1.96 (1.27 to 3.03), P = 0.002
-- Ever used psychoactive drug: HR 1.46 (1.05 to 2.02), P = 0.024
-- Stopped ART before because of toxicity: HR 1.74 (1.25 to 2.42), P = 0.001
The ATHENA team concluded that adults in their cohort stopped DTG or EVG infrequently for any reason or for toxicity but that toxicity was the main reason for stopping.
Reference
1. Bollen P, Hakkers C, Boender TS, et al. Discontinuation of dolutegravir- and elvitegravir-containing cART in the Netherlands: incidence rates and risk factors. 16th European AIDS Conference. October 25-27, 2017. Milan. Abstract PE12/10.

|
| |
|
 |
 |
|
|