 |
 |
 |
| |
Hepatic Fibrosis is Associated With Histological Activity in Non-alcoholic Steatohepatitis:
an Analysis From a Large Database of Screening Biopsies in the CENTAUR Trial
|
| |
| |
Cenicriviroc Versus Placebo for the Treatment of Nonalcoholic Steatohepatitis with Liver Fibrosis: Results from the Year 1 Primary Analysis of the Phase 2b CENTAUR Study http://www.natap.org/2016/AASLD/AASLD_115.htm
Cenicriviroc Versus Placebo for the Treatment of Nonalcoholic Steatohepatitis with Liver Fibrosis: Results from the Year 1 Primary Analysis of the Phase 2b CENTAUR Study press release http://www.businesswire.com/news/home/20160725005384/en/Tobira-Therapeutics-Announces-Clinically-Statistically-Significant-Improvement
Antifibrotic activity of dual CCR5/CCR2 antagonist cenicriviroc in a mouse model of renal fibrosis.....http://www.natap.org/2014/IAC/IAC_106.htm
Final Week 48 Analysis of Cenicriviroc (CVC) Compared to Efavirenz (EFV), in Combination with Emtricitabine/Tenofovir (FTC/TDF), in Treatment-Naïve HIV-1-Infected Adults with CCR5-Tropic Virus (Study 652-2-202; NCT01338883)......http://www.natap.org/2013/EACS/EACS_10.htm
Week 24 Primary Analysis of Cenicriviroc vs Efavirenz, in Combination with FTC/TDF, in Treatment-naïve HIV-1 Infected Adults with CCR5-tropic virus....http://www.natap.org/2013/CROI/croi_41.htm
Anti-Fibrotic and Anti-Inflammatory Activity of the Dual CCR2 and CCR5 Antagonist Cenicriviroc in a Mouse Model of NASH....http://www.natap.org/2013/AASLD/AASLD_123.htm
The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomized trial - http://www.natap.org/2016/HIV/021916_06.htm
"In conclusion, 5 mg CCX140-B once a day lowers albuminuria in patients with type 2 diabetes and proteinuria, on top of standard of care, and without any marked side-effects. These results suggest that CCX140-B is a promising candidate for further clinical development to reduce the unmet need for treatments for diabetic renal disease. - Repeated measures analysis showed a significant least squares mean change from baseline in albuminuria for CCX140-B 5 mg (figure 2): -18% (95% CI -26% to -8%; p=0⋅0004) compared with placebo -2% (95% CI -11% to 9%; p=0⋅72) during 52 weeks; the difference between 5 mg CCX140-B and placebo was -16% (one-sided 95% upper confidence limit -5%; p=0⋅01). The 10 mg dose showed a -11% (95% CI -20% to -1%; p=0⋅02) least squares mean change from baseline during 52 weeks; -10% (upper 95% confidence limit 2%) compared with placebo (p=0⋅08).
Pharmacokinetics of cenicriviroc when administered with and without ritonavir, darunavir/ritonavir or atazanavir/ritonavir. - Protease Inhibitors Boost Levels of Cenicriviroc, a CCR5/CCR2 Antagonist - http://www.natap.org/2013/Pharm/Pharm_11.htm
Cenicriviroc -
CROI REPORT: New Drugs for HIV Treatment and other random thoughts written for NATAP by Joe Eron MD as part of CRO 2013 coverage for new HIV drugs
http://www.natap.org/2013/CROI/croi_116.htm
Cenicriviroc is the fourth CCR-5 inhibitor to make it into later stage clinical development. Only one, maraviroc, completed clinical development and has modest use in clinical practice in the US and in Europe. Cenicriviroc is a clear once daily CCR-5 receptor antagonist with a 30+ hour half-life and activity in early phase clinical trial comparable to the other CCR-5 inhibitors. What makes cenicriviroc more interesting than the potential to be a second in class agent is the fact that this agent also inhibits binding of MCP-1 (monocyte chemoattratant protein one) to the CCR-2 receptor. CCR-2 is expressed on monocytes/macrophage and binding with its ligands, such as MCP-1, results in activation of this cell type. MCP-1 in expressed on endothelial cells, stromal cells and other inflammatory cells and the receptor ligand interaction can lead to increased inflammation in the setting of tissue injury and this interaction may be a mediator of inflammatory diseases including atherosclerosis (8). Even at this meeting an association between monocyte activation and coronary artery calcium was observed in HIV-1 infected individuals (13, 14). Monocyte/macrophage activation and resultant inflammation may be an important component of the inflammatory state in untreated and treated HIV-1 infection and an antiretroviral that may also impact ongoing inflammation has a particular attraction.
There are several caveats. The first is to demonstrate that cenicriviroc has sufficient activity as an antiretroviral to warrant further development. Joe Gathe and colleague presented the week 24 phase IIb data from a comparative trial of cenicriviroc (100 or 200 mg QD) plus TDF/FTC compared to EFV plus TDF/FTC (2;2;1 randomization) in treatment naïve patients who had R5 virus (10). Baseline HIV RNA had to be greater than 1,000 c/mL and CD4 at baseline was greater than 200 cells/mm3. The EFV and CVC were fully blinded so each patient had to take 6 pills daily because at the start of the study CVC was available in 50 mg tablets. Frankly the results were mixed. Despite a relatively low median baseline viral load of approximately 30,000 c/mL and a baseline CD4 cell count of approximately 400 cells/mm3, 76, 73 and 71% of subjects on the 100 mg CVC, 200 mg CVC and EFV arms respectively, were < 50 c/mL by the FDA snapshot analysis. Clearly these results demonstrate a comparable outcome. However, the reasons for treatment failure at week 24 varied by the third agent. Only 1 out of 28 (4%) EFV treated patients had virologic non-response where as most of the treatment failure was explained by discontinuation due to AE, discontinuation due to non AE, non virologic reasons such as lost to follow-up and withdrawal of consent. In somewhat of a contrast 12 and 13% of subjects on the 100 mg and 200 mg CVC arms had virologic non-response. Non-AE withdrawal were also high; 10 and 11% respectively. Essentially only one patient in the two CVC arms discontinued due to an AE.
The proportion of protocol defined virologic failure was more similar across arms with 12%, 16% and 11% meeting the protocol definition of virologic failure in the 100 mg CVC, 200 mg CVC and EFV arms, respectively. Protocol defined virologic failure required that a confirmatory viral load be > 400 c/mL so low level viremia > 50 c/mL but < 400 c/mL presumably would not be declared VF. Five of 13 patients with amplifiable virus on the CVC arms develop NRTI resistance mutations. One of six patients who had viral tropism assessed had emergence of a dual mixed virus. CD4 responses were quite similar. There were a greater number of grade 2 or greater clinical AE in the EFV arm and, as mentioned, a substantially greater percentage of EFV-treated patients stopped therapy due to an AE. Grade 3/4 laboratory abnormalities were quite similar between arms with the exception of a greater percentage of grade 3 and 4 CPK rises in the 200 mg CVC arm. Total cholesterol and LDL levels in the CVC arms actually fell, while they rose measurably in the EFV arm.
So one could ask the question was the first caveat met? Did CVC show enough activity/efficacy in the standard 2 NRTI plus backbone drug background format to warrant phase III study. This is a tough call. There certainly are suggestions in the data that CVC may not have had the same antiretroviral potency compared to the EFV-based regimen including perhaps some greater vulnerability in the higher viral load strata. However, several virologic failures had only low level viremia and CVC certainly appeared to be well tolerated at either dose over the early (24 week) segment of this study. CVC is neither and inducer or inhibitor of cyp3A4 and novel combinations in first line therapy could be considered and there appears to be enough signal here to warrant phase III study.
The second caveat is - Does the inhibition of the CCR-2 receptor add important clinical value? The study clearly showed dose-dependent rises in MCP-1 indicative of receptor-ligand blockage. The IC50 for the CCR-2 antagonism is roughly 20-fold higher than the IC90 for HIV-1 inhibition via CCR-5 blockage so the fold increase using the same metric (either IC50 or IC90) for both would likely lead to an even higher ratio. No clear dose dependence was seen in the anti-HIV activity was seen so dose selection will be interesting. I think this study does prove the CVC antagonizes the MCP-1:CCR-2 ligand:receptor interaction in vivo. Proving there is a clinical benefit to blocking this interaction will be a more substantial challenge but one that should clearly be pursued as the results of the phase IIb study don't really suggest that there will be dramatic advantages over maraviroc other than the once daily dosing and the potential for a clinically meaningful blockage of the MCP-1 CCR-2 interaction. Also CCR-2 blockade is not without, at least, theoretical risk as monocyte/macrophage activation may be important for the killing of certain intracellular organisms. Dose selection and the design of future studies to establish substantial HIV activity and some clinically favorable outcome related to CCR-2 antagonism will be interesting to see.
CROI: Week 24 Primary Analysis of Cenicriviroc vs Efavirenz, in Combination with FTC/TDF, in Treatment-naïve HIV-1 Infected Adults with CCR5-tropic virus - (03/08/13)
Reported by Jules Levin
EASL April 19-23 2017
Zachary Goodman,1 Vincent Wai-Sun Wong,2 Nicolas Lanthier,3 Jerôme Boursier,4 William Alazawi,5 Johannes Kluwe,6 Joan Genescà,7 Pietro Andreone,8 Lawrence Serfaty,9
Richard Skoien,10 Brian L. Wiens,11 Pamela Vig,11 Eric Lefebvre,11 Star Seyedkazemi,11 Zeid Kayali,12 Fred Poordad,13 Arun Sanyal,14 Frank Tacke,15 Vlad Ratziu16
1Inova Fairfax Medical Campus, Fall Church, VA, USA; 2The Chinese University of Hong Kong, Hong Kong, The People's Republic of China; 3Cliniques universitaires Saint-Luc, Brussels, Belgium; 4Angers University Hospital, Angers, France; 5The Blizard Institute, Queen Mary University of London, London, UK; 6Hamburg University Medical Center, Hamburg, Germany; 7Vall d'Hebron Research Institute, Barcelona, Spain; 8University of Bologna, Bologna, Italy; 9Saint-Antoine Hospital, Paris, France; 10Royal Brisbane and Women's Hospital, Herston, Queensland, Australia; 11Allergan plc, South San Francisco, CA, USA; 12Inland Empire Liver Foundation, University of California Riverside, Rialto, CA, USA; 13Texas Liver Institute/University of Texas Health, San Antonio, TX, USA; 14Virginia Commonwealth University, Richmond, VA, USA; 15University Hospital Aachen, Aachen, Germany; 16Pitie-Salpetrière Hospital and Pierre and Marie Curie University, Paris, France
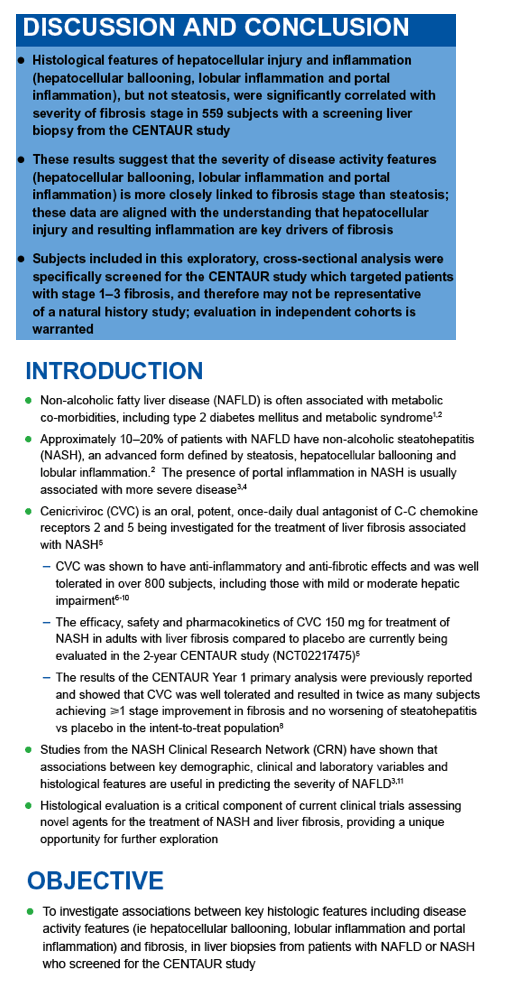

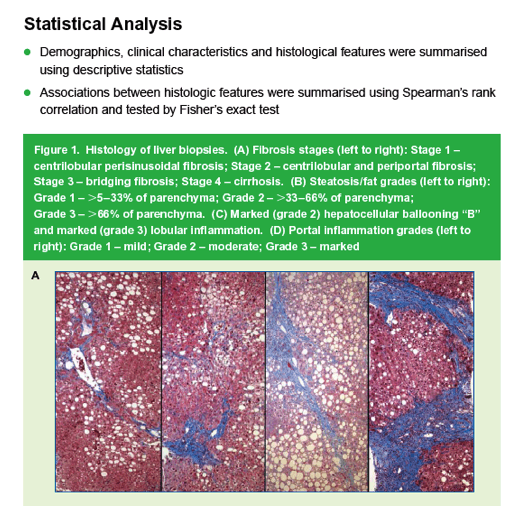
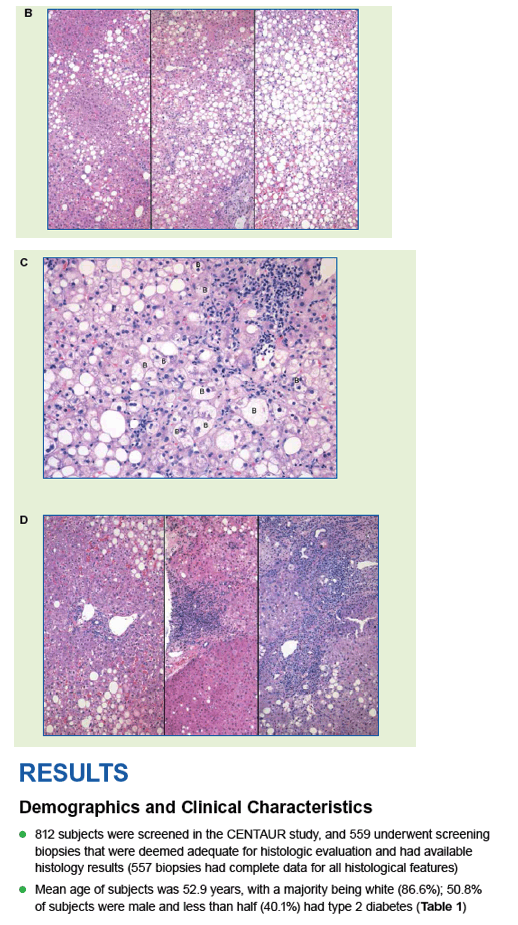

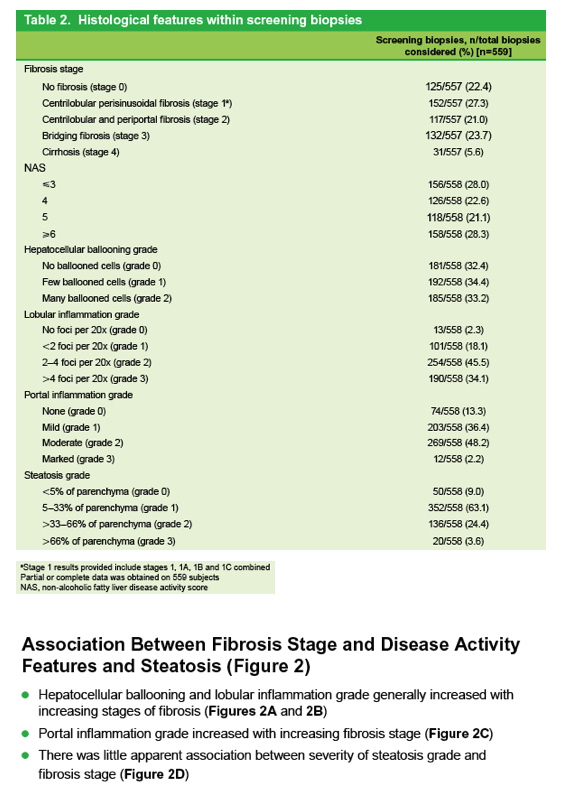
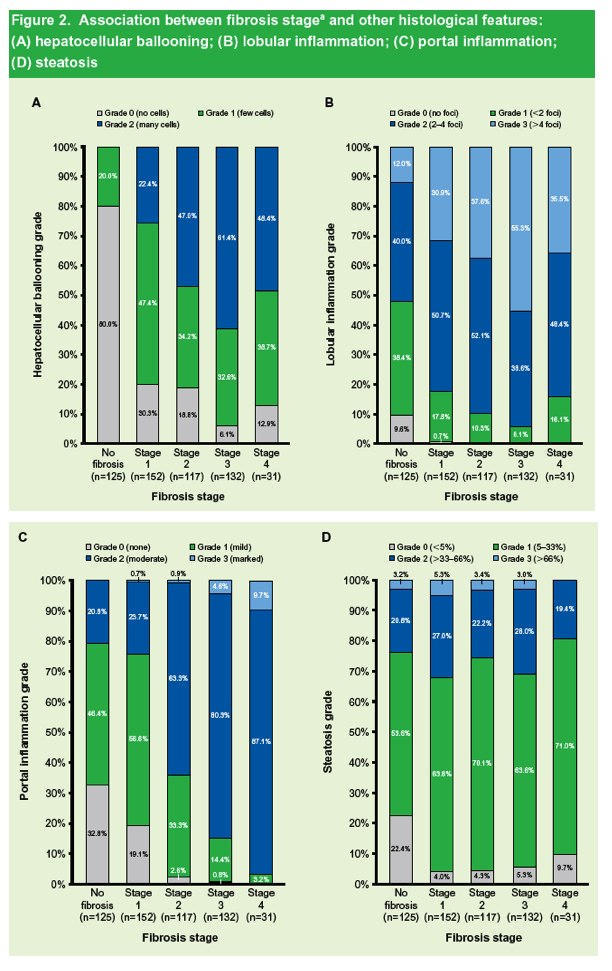
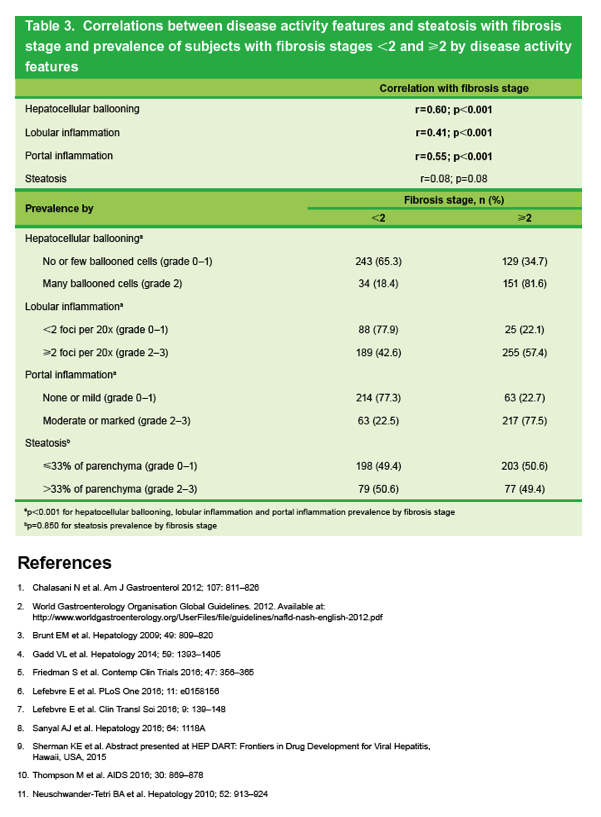
|
| |
|
 |
 |
|
|