| |
The association between race/ethnicity and the effectiveness
of direct antiviral agents for hepatitis C virus infection
|
| |
| |
Download the PDF here
Hepatology Feb 2017
Feng Su,1 Pamela K. Green,2 Kristin Berry,2 and George N. Ioannou1,2
Abstract
Black race and Hispanic ethnicity were associated with lower rates of sustained virologic response (SVR) to interferon-based treatments for chronic hepatitis C virus infection, whereas Asian race was associated with higher SVR rates compared to white patients. We aimed to describe the association between race/ethnicity and effectiveness of new direct-acting antiviral regimens in the Veterans Affairs health care system nationally. We identified 21,095 hepatitis C virus–infected patients (11,029 [52%] white, 6,171 [29%] black, 1,187 [6%] Hispanic, 348 [2%] Asian/Pacific Islander/American Indian/Alaska Native, and 2,360 [11%] declined/missing race or ethnicity) who initiated antiviral treatment with regimens containing sofosbuvir, simeprevir + sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ombitasvir/ritonavir/dasabuvir during the 18-month period from January 1, 2014, to June 30, 2015. Overall SVR rates were 89.8% (95% confidence interval [CI] 89.2-90.4) in white, 89.8% (95% CI 89.0-90.6) in black, 86.0% (95% CI 83.7-88.0) in Hispanic, and 90.7% (95% CI 87.0-93.5) in Asian/Pacific Islander/American Indian/Alaska Native patients. However, after adjustment for baseline characteristics, black (adjusted odds ratio = 0.77, P < 0.001) and Hispanic (adjusted odds ratio = 0.76, P = 0.007) patients were less likely to achieve SVR than white patients, a difference that was not explained by early treatment discontinuations. Among genotype 1–infected patients treated with ledipasvir/sofosbuvir monotherapy, black patients had significantly lower SVR than white patients when treated for 8 weeks but not when treated for 12 weeks. Conclusion: Direct-acting antivirals produce high SVR rates in white, black, Hispanic, and Asian/Pacific Islander/American Indian/Alaska Native patients; but after adjusting for baseline characteristics, black race and Hispanic ethnicity remain independent predictors of treatment failure. Short 8-week ledipasvir/sofosbuvir monotherapy regimens should perhaps be avoided in black patients with genotype 1 hepatitis C virus.
LDV/SOF, PrOD, SMV+SOF, and SOF-based antiviral regimens resulted in high SVR rates in all racial/ethnic groups among 21,095 veterans with HCV treated in the VA national health care system in 2014 and 2015. However, after adjustment for baseline characteristics, black (AOR = 0.77, P < 0.001) and Hispanic (AOR = 0.76, P = 0.007) patients were less likely to achieve SVR than white patients, a difference that was not explained by early treatment discontinuations. Among genotype 1–infected patients treated with LDV/SOF monotherapy, black patients had significantly lower SVR than white patients when treated for 8 weeks but not when treated for 12 weeks.
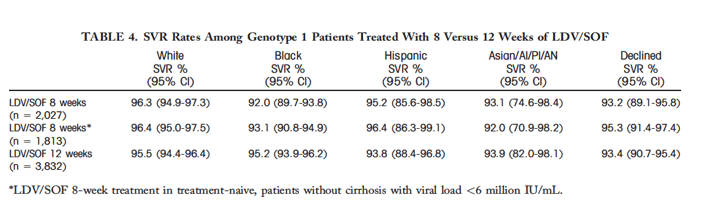
LDV/SOF TREATMENT FOR 8 WEEKS IN GENOTYPE 1 PATIENTS AND ASSOCIATION WITH SVR
Food and Drug Administration guidelines and the LDV/SOF package insert suggest that 8 weeks of LDV/SOF monotherapy may be considered among genotype 1–infected patients who are treatment-naive, do not have cirrhosis, and do have a viral load <6 million IU/mL[37]; however, this regimen is based on a post hoc analysis of the ION-3 study,[3] and it is unclear if it is widely used. Among 8,140 patients treated with LDV/SOF monotherapy, a similar proportion of white (28.7%), black (26.3%), and Asian/PI/AI/AN (27.6%) patients received 8 weeks of therapy compared to 20.3% of Hispanic patients. SVR rates were very similar in white, Hispanic, and Asian/PI/AI/AN patients who received 8 and 12 weeks of therapy; but they were slightly lower in blacks who received 8 weeks (92.0%, 95% CI 89.7-93.8) versus 12 weeks (95.2%, 95% CI 93.9-96.2) (Table 4). Also, when limiting to treatment-naive patients without cirrhosis with a viral load <6 million who received 8 weeks of LDV/SOF, SVR rates were lower in black (93.1%) than in white (96.4%) or Hispanic (96.4%) patients. In multivariate analysis, black race was associated with lower likelihood of SVR among patients who received 8 weeks of therapy (AOR = 0.56, 95% CI 0.36-0.88) but not among patients who receive 12 weeks of therapy (AOR = 0.89, 95% CI 0.63-1.27) (Table 5).
EARLY TREATMENT DISCONTINUATION BY RACIAL/ETHNIC GROUPS
Among all patients who initiated treatment (n = 21,095), early discontinuation of treatment in <8 weeks was slightly more common in black (7.3%), Hispanic (7.9%), and Asian/PI/AI/AN (7.2%) patients than in white patients (5.8%) (Supporting Table S1). Mean duration of treatment was 88 days in white, 83 days in black, 89 days in Hispanic, and 87 days in Asian/PI/AI/AN patients. Among patients with available SVR data (n = 19,286), whose SVR results are shown in Tables 2 and 3, early treatment discontinuation in <8 weeks occurred in 4.3% of white, 5.4% of black, 6.3% of Hispanic, and 5.3% of Asian/PI/AI/AN patients.
Discussion
LDV/SOF, PrOD, SMV+SOF, and SOF-based antiviral regimens resulted in high SVR rates in all racial/ethnic groups among 21,095 veterans with HCV treated in the VA national health care system in 2014 and 2015. However, after adjustment for baseline characteristics, black (AOR = 0.77, P < 0.001) and Hispanic (AOR = 0.76, P = 0.007) patients were less likely to achieve SVR than white patients, a difference that was not explained by early treatment discontinuations. Among genotype 1–infected patients treated with LDV/SOF monotherapy, black patients had significantly lower SVR than white patients when treated for 8 weeks but not when treated for 12 weeks.
Our results represent a dramatic deviation from the interferon-based antiviral treatments, which consistently reported much larger gaps in SVR between white patients and black or Hispanic patients, in both clinical trials and real-world studies.[21] The narrowing of the SVR gap between black and white patients may be related to the fact that the efficacy of DAA-based regimens does not appear to be dependent on interleukin-28B gene (IL28B) polymorphisms, which strongly influence response to interferon. Disparities between black and white patients in treatment responses were in part related to the lower prevalence of the CC allele of the IL28B gene in black patients, which is associated with higher rates of SVR in response to interferon-based regimens.[38] In clinical trials of DAA regimens, however, SVR rates are very similar between IL28B CC and non-CC patients.[1, 3-8, 39-41]
Racial/ethnic "minority" groups, such as blacks and Hispanics, are underrepresented in antiviral treatment clinical trials, despite the fact that black patients and some Hispanic groups, such as Puerto Ricans, are overrepresented among HCV-infected patients. Thus, it is frequently unclear whether the results of clinical trials that are based mostly on white patients will apply to racial/ethnic "minority" groups in real-world clinical practice. Our results offer some reassurance that black and Hispanic patients achieve SVR rates comparable to those of white patients in real-world clinical practice, although small gaps still exist.
Some differences in baseline characteristics by racial/ethnic group are important to highlight and inform the interpretation of differences in SVR rates. First, the prevalence of genotype 2 or 3 HCV infection was dramatically lower in black patients (3.0% and 0.7%, respectively) than other racial/ethnic groups. These differences in genotype distribution by race have been reported.[12-16] Genotypes 2 and 3 were regarded as "favorable" in the interferon era but are now associated with the lowest SVR rates in response to DAA therapy. It is therefore critical to stratify or adjust for genotype when comparing different racial groups. Second, the prevalence of cirrhosis was lower in black patients (30.7%) and higher in Hispanic patients (48.7%) compared to white (35.2%) or Asian/PI/AI/AN (35.1%) patients (Table 1). The differences in prevalence of cirrhosis by race/ethnicity are consistent with previous reports[42, 43] and were mirrored in the proportions with elevated FIB-4 scores, elevated serum bilirubin levels, or reduced platelet count (Table 1). It has been speculated that the higher prevalence in Hispanics and the lower prevalence in blacks of fatty liver disease, visceral obesity, and insulin resistance contribute to the corresponding risk of cirrhosis among HCV-infected patients.[42, 43] Cirrhosis is associated with lower SVR rates and therefore has to be adjusted for when investigating the associations between race/ethnicity and SVR. Indeed, adjustment for genotype and cirrhosis is responsible for "reversing" the unadjusted odds ratio in black patients from a value slightly greater than 1 (i.e., more likely to respond) to an AOR <1 (i.e., less likely to respond) (Table 3).
We explored whether the disparity between white, black, and Hispanic patients could be related to different rates of early treatment discontinuation. With interferon-based regimens, rates of early discontinuation were very high but similar between black and white patients[16, 33] and therefore did not contribute to the racial gap in SVR. Rates of early discontinuation of DAA regimens in our study are much lower than what has been observed with interferon. Although early discontinuation was slightly more common in black and Hispanic patients compared to white patients, when we adjusted for duration of antiviral treatment (which accurately captures early discontinuations), there was minimal impact on the AORs for the association between race/ethnicity and SVR. Thus, differences in early discontinuation of treatment do not account for the association between race/ethnicity and SVR that we identified in multivariable analyses.
In the ION-4 clinical trial of HIV/HCV coinfected patients treated with 12 weeks of LDV/SOF monotherapy, SVR rates were significantly lower in black (90%, 95% CI 83-95) than in white (99%, 95% CI 97-100) patients.[9] We also found a difference in SVR between black (90.3%, 95% CI 87.1-92.7) and white (93.9%, 95% CI 89.2-96.6) patients, but it was smaller and did not reach statistical significance in either crude or adjusted analyses. Limiting to genotype 1–infected patients who received LDV/SOF monotherapy, as in the ION-4 trial, black patients again had a lower SVR (92.0%, 95% CI 88.3-94.7) than white patients (95.1%, 95% CI 87.3-98.1), which was nonsignificant.
It is recommended that a short, 8-week LDV/SOF monotherapy regimen "can be considered,"[44] "with caution and at the discretion of the practitioner,"[37] in treatment-naive, genotype 1–infected patients without cirrhosis with an HCV viral load <6 million. Indeed, these 8-week regimens were commonly used in the VA and resulted in high overall SVR rates.[45] However, our results as well as other recent VA studies,[46] show that black patients had significantly lower SVR than white patients when treated with 8 weeks but not when treated with 12 weeks of LDV/SOF. Furthermore, recent pooled analyses of data from the ION-1, ION-2, and ION-3 clinical trials, which evaluated the efficacy of LDV/SOF with or without RIBA for treatment of genotype 1 HCV infection, reported that among patients treated with LDV/SOF monotherapy for 8 weeks, the relapse rate was higher (7/81 or 8.6%) and the SVR rate lower (91.3%) in black patients than nonblack patients (relapse rate 13/348 [3.7%] and SVR rate 96.2%).[20, 47] Collectively, these results suggest that the 8-week regimens should perhaps be avoided in black patients and are in agreement with the most recent combined guidelines of the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America,[44] which suggest that "shortening treatment to less than 12 weeks is not recommended for HIV-infected patients, African-American patients, or those with known IL28 polymorphism CT or TT."
In multivariate models, patients in the Asian/PI/AI/AN race/ethnicity group did not have a statistically significant difference in likelihood of SVR compared to white patients (Table 3). Unadjusted SVR rates in Asian/PI/AI/AN patients were also similar to other groups (Table 2). This is a slight departure from trends seen with interferon-based treatments, which typically produced significantly higher rates of SVR in Asian patients than in white, black, and Hispanic patients.[18, 48] Asian patients have a higher frequency of the favorable CC IL28B allele,[38] partly accounting for better response to interferon-based treatments. The elimination of the SVR gap between Asian patients and white patients may be due to the lack of impact of the IL28B genotype on response to DAA-based regimens.
An important limitation of our study is that a significant proportion (12%) of patients had a missing or declined race/ethnicity designation. This could potentially have biased our results if one race/ethnicity group was more likely than others to have a missing race designation, leading to a high proportion of missing results for that particular group. However, the baseline characteristics and SVR rates of patients with missing race/ethnicity data did not mirror any one particular race group and instead were generally an average of all the race groups. It is therefore unlikely that the missing data biased our results in any particular direction.
Also, our study is limited by missing SVR data in 8.6% of patients, which may lead to overestimated SVR rates among those with available SVR data, if those with missing SVR data are significantly less likely to have achieved SVR. We think this is unlikely for two reasons. First, patients with missing SVR data were very similar to those with available SVR data in baseline characteristics that predict SVR (Supporting Table S2). Although early discontinuation of treatment in <8 weeks was more common in patients with missing SVR data (24.8% versus 4.4%), the majority of patients with missing SVR completed 8 or more weeks of treatment, demonstrating that patients with missing SVR data were not patients who "dropped out" of treatment or were "lost to follow-up" but rather patients (or physicians) who were simply delinquent in getting their SVR viral load measured after the end of their treatment-not an uncommon phenomenon outside of clinical trials. Second, we used comprehensive multiple imputation models that included duration of treatment in addition to baseline, pretreatment characteristics to impute the missing SVR data and found only an insubstantial reduction in SVR after imputation (Table 4), suggesting that it is unlikely that our results of observed SVR are biased toward overestimation due to the missing SVR data.
Our results demonstrate that DAA-based regimens are highly effective for treatment of chronic HCV among all race and ethnicity groups in real-world practice. Although black race and Hispanic ethnicity are still associated with lower likelihood of SVR in multivariate analysis, DAAs hold promise in closing the SVR gap between different race/ethnicity groups. Future studies of HCV treatment regimens should ensure adequate inclusion of racial/ethnic minorities in study populations to better detect differences in clinical subgroups.
|
|
| |
| |
|
|
|