| |
Expansion of Treatment for Hepatitis C Virus Infection by Task Shifting to Community-Based Nonspecialist Providers: A Nonrandomized Clinical Trial - ASCEND Study
|
| |
| |
FDA Vosevi Package Insert - Download the PDF here
Nonrandomized Clinical Trial - ASCEND Study
8 August 2017
"In this phase 4 prospective, observational study, task shifting of DAA-based HCV therapy to nonspecialist providers was safe and effective. In the primary outcome analysis, no significant difference in SVR was observed among patients treated by specialists, PCPs, and NPs. In addition, the trial outcomes parallel industry-sponsored registration trials, in a real-world urban cohort with a high prevalence of HCV, HIV, and poverty (16).
Our study was conducted within the setting and time limitations of standard medical practice, without the use of patient navigators, in FQHCs serving an urban, socioeconomically challenged population, thus demonstrating the feasibility of delivering DAA treatment in these existing sites. Treatment was safe, with no deaths related to study participation and with an AE profile similar to that of previous LDV-SOF studies. Finally, this system of care was implemented after a 3-hour guideline-based training session. Taken together, these results support decentralization of HCV treatment to facilitate greater therapeutic capacity for the many patients who remain untreated."
"Currently in the United States, only 50% of patients with HCV infection are aware of their diagnosis (19), and given the national recommendations for expanded screening (20, 21), as well as consensus guidance (22) for treatment in all but those with a short life expectancy, therapeutic demand likely will continue to increase.
Although ASCEND included a small number of providers limited to 2 clinic systems, the results suggest that in the DAA era, nonspecialist providers can be trained rapidly to offer a single-step "diagnosis-linkage-treatment" continuum, avoiding the need for referrals in uncomplicated cases.
Among 551 patients with available prescription adherence data, the vast majority (87%) picked up all prescribed medication.....Although SVR is not a direct measure of medication adherence, our analysis found that patients with 100% prescription adherence had a higher SVR rate than those with less than 100% adherence, despite moderate rates of attendance to provider visits.....Among 551 patients with prescription adherence data, 477 (86.6%) had 100% adherence, defined as picking up all prescribed medication. Of these 477 patients, 427 (89.5%) achieved SVR, whereas among 74 patients with less than 100% prescription adherence, only 46 (62.2%) achieved SVR. Patients with an SVR had a higher mean percentage of prescription adherence than those without an SVR (96.3% vs. 82.7%)...7.5% of patients were LTFU during the study, an attrition rate consistent with that of other real-world investigations and reflective of the complex socioeconomic dynamics of this urban cohort. Together with adherence data, these findings support identification of patients most at risk for nonadherence, as well as counseling regarding the meaning of SVR and the importance of testing for it. A recent study of a validated assessment tool (23) suggests that such processes need not be time or resource intensive.
ASCEND had several strengths, foremost its inclusion criteria, which paralleled the LDV-SOF label. As such, the study population is generalizable to persons living with HCV infection in U.S. cities, where the prevalence of comorbid conditions, including substance use disorder and polypharmacy, is substantial. To our knowledge, the ASCEND cohort is the largest single study to date of LDV-SOF therapy in black patients.
Of importance, this investigation differs from real-life practice in that all providers dispensed medication directly to patients at the treating facility, avoiding the rigorous process of prior authorization-including restrictions regarding provider type-currently required by public and private insurance plans and managed care organizations in most states. Because of these requirements, most PCPs and NPs in the United States currently are not permitted to provide independent HCV treatment. The ASCEND investigation suggests that such provider restrictions are not supported by evidence and stand as unnecessary hurdles in the HCV care continuum. Reversal of such policies might allow rapid escalation of safe, effective therapy for HCV infection and improve the care of patients living with this potentially fatal disease."
--------------------------------------
8 August 2017
Expansion of Treatment for Hepatitis C Virus Infection by Task Shifting to Community-Based Nonspecialist Providers: A Nonrandomized Clinical Trial
Annals of Int Medicine - Sarah Kattakuzhy, MD; Chloe Gross, RN; Benjamin Emmanuel, MPH; Gebeyehu Teferi, MD; Veronica Jenkins, MD; Rachel Silk, RN, MPH; Elizabeth Akoth, RN, MS; Aurielle Thomas, BA; Charisse Ahmed, BS; Michelle Espinosa; Angie Price, CRNP;
Elana Rosenthal, MD; Lydia Tang, MD; Eleanor Wilson, MD, MS; Soren Bentzen, PhD; Henry Masur, MD; Shyam Kottilil, MD, PhD;
and the ASCEND Providers*
Abstract
Background:
Direct-acting antiviral (DAA) therapy for hepatitis C virus (HCV) infection has resulted in high rates of disease cure; however, not enough specialists currently are available to provide care.
Objective:
To determine the efficacy of HCV treatment independently provided by nurse practitioners (NPs), primary care physicians (PCPs), or specialist physicians using DAA therapy.
Design:
Nonrandomized, open-label clinical trial initiated in 2015. (ClinicalTrials.gov: NCT02339038)
Setting:
13 urban, federally qualified health centers (FQHCs) in the District of Columbia.
Patients:
A referred sample of 600 patients, of whom 96% were black, 69% were male, 82% were treatment naive, and 20% had cirrhosis. Seventy-two percent of the patients had HCV genotype 1a infection. The baseline characteristics of patients seen by each provider type were similar.
Intervention:
Patients were assigned in a nonrandomized but specified manner to receive treatment from 1 of 5 NPs, 5 PCPs, or 6 specialists. All providers underwent an identical 3-hour training session based on guidelines. Patients received treatment with ledipasvir-sofosbuvir, which was provided on site, according to U.S. Food and Drug Administration labeling requirements.
Measurements:
Sustained virologic response (SVR).
Results:
516 patients achieved SVR, a response rate of 86% (95% CI, 83.0% to 88.7%), with no major safety signals. Response rates were consistent across the 3 provider types: NPs, 89.3% (CI, 83.3% to 93.8%); PCPs, 86.9% (CI, 80.6% to 91.7%); and specialists, 83.8% (CI, 79.0% to 87.8%). Patient loss to follow-up was the major cause of non-SVR.
Limitation:
Nonrandomized patient distribution; possible referral bias.
Conclusion:
In a real-world cohort of patients at urban FQHCs, HCV treatment administered by nonspecialist providers was as safe and effective as that provided by specialists. Nurse practitioners and PCPs with compact didactic training could substantially expand the availability of community-based providers to escalate HCV therapy, bridging existing gaps in the continuum of care for patients with HCV infection.
Primary Funding Source:
National Institutes of Health and Gilead Sciences.
The recent introduction of highly effective, well-tolerated direct-acting antiviral (DAA) therapy for hepatitis C virus (HCV) infection has raised the possibility of rapid treatment expansion and widespread cure. Despite this scientific breakthrough, of the 43% of patients aware of their HCV diagnosis and linked to care, only 16% have begun treatment (1). With an estimated 20 000 gastroenterology-hepatology and infectious disease physicians in the United States (2), the current specialist workforce (3, 4) is insufficient to meet the treatment demands of the 2.7 million Americans living with HCV infection (5).
Several studies of partial task shifting-shared treatment between specialists and primary care providers-demonstrated its success in improving access to HCV care (6-11). Project ECHO (Extension for Community Healthcare Outcomes) (4) used a model of primary care physician (PCP)-based HCV treatment with specialist mentorship to demonstrate equivalent cure rates between PCPs and specialists. However, few of these studies were prospective or comparative, and all used interferon-based regimens, limiting their applicability to current practice. In the DAA era, complete task shifting of HCV therapy to general practitioners may be the ideal strategy for patients with uncomplicated infections (12, 13). Yet, information on the success of nonspecialists practicing independent of specialist supervision is limited.
The primary objective of ASCEND (A Phase IV Pilot Study to Assess Community-Based Treatment Efficacy in Chronic Hepatitis C Monoinfection and Coinfection With HIV in the District of Columbia) was to evaluate the efficacy of HCV treatment managed independently by 3 community-based provider types-nurse practitioners (NPs), PCPs, and specialists-after a succinct, guideline-driven educational intervention, and set within a real-world, urban population.
Methods
Trial Design
In this prospective, open-label, observational clinical trial at 13 community health centers in the District of Columbia, 600 patients with chronic HCV infection were assigned in a nonrandomized fashion to receive treatment with ledipasvir (LDV) and sofosbuvir (SOF) according to label from 1 of 3 provider types: a licensed NP, a PCP (defined as a physician board-certified in family or internal medicine), or a specialist (defined as an internist specializing in infectious diseases or gastroenterology-hepatology).
Sites
Participating federally qualified health centers (FQHCs) in Washington, DC, provide care to a primarily African American, publically insured, underserved population. Twelve of these clinics are part of a clinical network providing primary and subspecialty care as well as social services. The infectious disease team within this network is an established group of specialist providers who rotate among various clinic sites to provide care to patients with infectious diseases, including HIV and HCV. The other site is an independent clinic that provides comprehensive health and social services. Study-specific templates were developed in eClinicalWorks, the electronic medical record common to all involved sites.
Providers and Provider Training
Sixteen providers (6 specialists [5 infectious disease physicians and 1 hepatologist], 5 PCPs, and 5 NPs) completed a uniform 3-hour training course before study initiation. The instruction focused on the following areas: HCV epidemiology and pathophysiology, screening, assessment of liver fibrosis, management of HIV-HCV co-infection, and post-sustained virologic response (SVR) care; Recommendations for Testing, Managing, and Treating Hepatitis by the American Association for the Study of Liver Diseases and Infectious Diseases Society of America (January 2015), on which study visit flow and monitoring were based; LDV-SOF, including dosing, pharmacology, and a review of potential drug interactions, particularly with antiretroviral therapy; and protocol-specific education on inclusion and exclusion criteria, prohibited medications, visit flow and monitoring, appropriate documentation, reporting, and oversight.
Patient Distribution
All participants were seen at their center at least once in the previous 5 years and were referred for the study by a health care provider. Patients were linked to providers in a nonrandomized but specified format. Approximately half of the patients were selected by the referring specialist provider to stay in treatment with him or her. The remaining half were distributed between NPs and PCPs on the basis of the following priorities:
Patient-provider relationship: If a patient had primary care services with a participating NP or PCP, he or she retained care with that provider.
Geographic location: If a patient did not have primary care services with a participating NP or PCP, he or she was treated by a participating provider at the patient's home clinic location or closest site.
Balance by HIV co-infection: Providers in all 3 categories were required to treat patients with HCV monoinfection as well as those with HIV-HCV co-infection. As such, some patients were distributed to treatment by an NP or PCP to ensure adequate numbers of patients for each provider type.
Patients per provider: Because some clinic locations had more than 1 provider type at each site, patients were distributed to ensure an approximate balance of patient load per provider.
The distribution criteria were designed by the study team in conjunction with principal investigators of the FQHCs to parallel real-world practice while preserving clinic function and study outcomes. Additional information regarding specific study conduct may be found in the Supplement.
Study Population
The study enrolled 600 patients between 20 January 2015 and 24 November 2015 at 13 FQHCs (Figure 1). Eligible patients were older than 18 years and had confirmed chronic genotype 1 HCV infection (3). Patients were excluded if they were pregnant or were breastfeeding, had a diagnosis of hepatocellular carcinoma or decompensated liver disease, had an estimated glomerular filtration rate less than 30 mL/min/1.73 m2, were receiving medications contraindicated with LDV-SOF (14), or could not provide informed consent. All patients had staging via liver biopsy, serologic biomarker test, or aspartate aminotransferase-to-platelet ratio index within 3 years of the screening visit. All 600 patients received and started LDV-SOF treatment without ribavirin between 14 May 2015 and 24 November 2015.
Study Visits and Assessments
The study team screened patients and obtained informed consent from those eligible to participate. Patients then were assigned to a treating provider and given a follow-up appointment. Beyond the visit on day 0, the study team played no further role in patient care and did not interact with the treating providers. The providers were instructed to contact the study team about any reportable adverse event (AE) findings, but for any clinical questions, they were to use the usual methods of their everyday practice. All follow-up assessments were completed at the discretion of the treating provider.
Patients were scheduled for once-a-month treatment visits with their provider; these visits were scheduled to coincide with blood draws for laboratory safety monitoring at week 4 and assessment of viral load at week 4 and at SVR. Providers prescribed LDV-SOF in 4-week increments via an in-house dispensing order within eClinicalWorks, which prompted nursing or administrative staff to obtain the study drug kept on site. The medication then was dispensed directly to the patient at the treating clinic location. Patients also could pick up medications at the treating clinic location if their prescription was due for a refill, even if they missed provider visits.
Patients were reminded of provider visits through the health center's usual mechanism: automated telephone reminders. No patient navigators were used.
End Points
The primary efficacy end point was SVR, defined as an undetectable HCV RNA viral load 12 weeks after treatment completion. All enrolled patients who received at least 1 dose of LDV-SOF were included in the final analysis, and patients lost to follow-up (LTFU) were considered to have treatment failure. Patients with detectable HCV RNA at the SVR time point were considered to have viral relapse. Secondary end points included evaluation of efficacy by subgroups: provider type, treatment duration, HIV serostatus, liver fibrosis stage, and cirrhosis. Cirrhosis was defined as a biopsy or serologic score of F4 or an aspartate aminotransferase-to-platelet ratio index score greater than 1.0. Exploratory end points included adherence to all treatment visits (a composite percentage, with the number of attended visits divided by the number of expected visits based on treatment duration) and to prescriptions (a composite percentage, with the number of prescriptions picked up divided by the number of expected prescriptions based on treatment duration). Patients who did not come to the clinic from 7 days before to 14 days after their scheduled treatment visit were considered to have a missed visit. Multiple longitudinal secondary outcomes are not reported here, because data collection is ongoing.
Safety Assessment
Adverse events, a secondary end point, were assessed by questioning and examinations, and each AE was assigned a grade from 1 to 4 according to Division of AIDS toxicity tables (15). Severe AEs and deaths were reported to the study team.
Study Oversight
The trial was sponsored by the National Institutes of Health Clinical Center; approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases; and conducted in compliance with the provisions of the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements.
Statistical Analysis
We set a sample size to estimate SVR with adequate precision. With 600 patients, the 2-sided 95% CI for SVR was expected to extend no more than 2.4% in both directions on the basis of a hypothesized 90% SVR. The primary analysis of efficacy was the proportion of overall patients who achieved SVR with a 2-sided 95% CI (based on the Clopper-Pearson method). A forest plot of the SVR rate (95% CI) was constructed for each of the 16 providers. An adjusted SVR rate (95% CI), both overall and by provider type, was constructed by using a generalized estimating equation model to account for clustering by provider. The secondary objective was to evaluate the efficacy by subgroup: provider type, treatment duration, HIV co-infection, liver fibrosis stage, and cirrhosis. During the study, 24 patients received HCV treatment from more than 1 provider type because of the standard of practice at each site in the case of provider absence. An additional analysis of the efficacy was based on recategorizing these patients from their original assigned provider type to a mixed provider type. Baseline demographic and clinical characteristics were compared among the 3 provider types by using 1-way analysis of variance for continuous variables and the chi-square or Fisher exact test for categorical variables.
Patients were considered LTFU if they had neither SVR outcome data nor a death report; in the primary analysis, they were considered to have treatment failure. Baseline characteristics were explored to determine whether any differences existed between patients with an SVR outcome and those LTFU or between patients LTFU during treatment and those LTFU after completing treatment.
All analysis was conducted in SAS, version 9.4 (SAS Institute). The forest plot was developed with Review Manager 5.3 (Cochrane). A P value less than 0.05 (2-sided) indicated statistical significance.
Role of the Funding Source
Gilead Sciences provided the study drug and collaborated on study design and analysis. All sponsors could provide comments on the written manuscript, but the primary and corresponding authors had the final decision regarding inclusion of edits and submission for publication.
Results
Baseline Characteristics of Study Patients
Six hundred patients were assigned to receive LDV-SOF from an NP (n = 150; 25%), a PCP (n = 160; 27%), or a specialist (n = 290; 48%). Patient characteristics are shown in Table 1. Overall, patients predominantly were male (69%), black (96%), and naive to HCV treatment (82%); did not have cirrhosis (80%), and had HCV genotype 1a infection (82%). Ninety percent of the patients were assigned to 12 weeks of LDV-SOF treatment by their provider. The baseline demographic and clinical characteristics of patients were similar among the 3 provider types, except for HIV co-infection (28% for PCPs, 24% for specialists, and 15% for NPs [P = 0.023]), race (blacks: 93% for NPs, 100% for PCPs, and 96% for specialists [P = 0.005]), and Hispanic ethnicity (1% for NPs, 0% for PCPs, and 3% for specialists [P = 0.038]).
Virologic Response
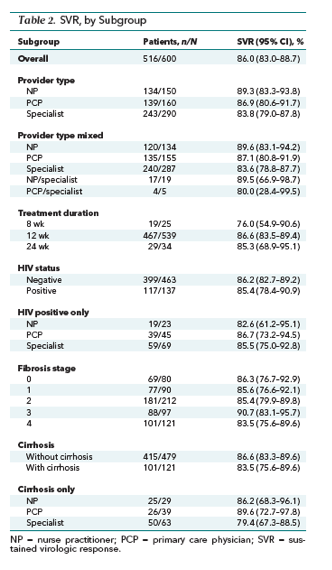
Of the 600 patients who received LDV-SOF, 86.0% (516) achieved SVR (95% CI, 83.0% to 88.7%). Of the 84 patients who did not achieve SVR, 45 (54%) were LTFU, 35 (42%) had viral relapse, and 4 (4%) died. The SVR rates among the 3 provider types were as follows: NPs, 89.3%; PCPs, 86.9%; and specialists, 83.8%.
The SVR rate for each subgroup is shown in Table 2, by provider type, mixed provider type, treatment duration, HIV co-infection, liver fibrosis stage, cirrhosis, and interferon treatment experience. Figure 2 illustrates the SVR forest plot for all 16 providers. After adjustment for age, sex, race, HIV status, and cirrhosis status and accounting for clustering by provider, the overall SVR rate was 87.1% (CI, 71.8% to 94.7%) and by provider type was as follows: NPs, 90.4% (CI, 59.0% to 98.4%); PCPs, 87.6% (CI, 62.0% to 96.8%); and specialists, 84.8% (CI, 70.2% to 93.0%).
Safety
A total of 98 participants had AEs: 96 (98%) had grade 1 or 2 events, most commonly fatigue (n = 45) and headache (n = 40). Eleven patients discontinued treatment early for medical reasons. Of these patients, 2 discontinued therapy on their own because of joint pain and malaise, and the other 9 had their HCV treatment stopped by the treating provider. In the latter group, 3 discontinuations were the result of severe headaches, generalized rash, and severe gastroesophageal reflux disease requiring an increase in proton-pump inhibitor dosage. The other 6 were the result of a grade-4 decrease in estimated glomerular filtration rate in patients with dual infection (5 with HIV and 1 with hepatitis B virus), 3 of whom were receiving tenofovir disoproxil-containing regimens.
Four deaths occurred during the study period, all unrelated to study participation. Two patients died of opioid overdose, and 2 died of autopsy-proven cardiovascular disease.
Loss to Follow-up
Forty-five patients (7.5%) were LTFU. The only significant difference in baseline characteristics between patients with an SVR (n = 551) and those LTFU (n = 45) was a younger age among the latter group (56.8 vs. 58.9 years; P = 0.047). Of patients LTFU, 17 (38%) were lost during treatment, whereas 28 (62%) were lost after completing treatment. There was no significant difference in baseline characteristics between patients LTFU during treatment and those LTFU after treatment.
HIV-HCV Co-infection
The SVR rate was 86.2% (CI, 82.7% to 89.2%) among HIV-negative patients and 85.4% (CI, 78.4% to 90.9%) in HIV-positive patients (Table 2). Among the 137 patients with HIV-HCV co-infection, 50% were assigned to specialists, compared with 33% assigned to PCPs and 17% to NPs. Among the HIV-positive patients only, SVR occurred in 82.6% of those seen by an NP, 86.7% by a PCP, and 85.5% by a specialist (Table 2).
Cirrhosis
An SVR was achieved by 83.5% (CI, 75.6% to 89.6%) of patients with cirrhosis and 86.6% (CI, 83.3% to 89.6%) of those without it (Table 2). Of the 121 patients with cirrhosis, 52% were assigned to specialists, whereas 24% were assigned to PCPs and 24% to NPs. Among the patients with cirrhosis, SVR occurred in 86.2% of those seen by an NP, 89.6% by a PCP, and 85.6% by a specialist (Table 2).
Treatment Visit and Prescription Adherence
The mean rate of adherence to treatment visits among all 600 patients was 62.2% (CI, 59.9% to 64.6%). Among the 539 patients who were assigned to receive 12 weeks of LDV-SOF treatment, visit adherence decreased over time: 76.4% (n = 412) attendance at week 4, 61.6% (n = 332) at week 8, and 50.5% (n = 272) at week 12. Adherence to treatment visits was lower among patients seen by a specialist (55.9% [CI, 52.6% to 59.3%]) than those seen by a PCP (63.1% [CI, 58.4% to 67.7%]) or an NP (73.6% [CI, 69.4% to 77.9%]) and higher among those who achieved SVR than those who did not (65.8% vs. 40.5%).
Among 551 patients with prescription adherence data, 477 (86.6%) had 100% adherence, defined as picking up all prescribed medication. Of these 477 patients, 427 (89.5%) achieved SVR, whereas among 74 patients with less than 100% prescription adherence, only 46 (62.2%) achieved SVR. Patients with an SVR had a higher mean percentage of prescription adherence than those without an SVR (96.3% vs. 82.7%).
Discussion
In this phase 4 prospective, observational study, task shifting of DAA-based HCV therapy to nonspecialist providers was safe and effective. In the primary outcome analysis, no significant difference in SVR was observed among patients treated by specialists, PCPs, and NPs. In addition, the trial outcomes parallel industry-sponsored registration trials, in a real-world urban cohort with a high prevalence of HCV, HIV, and poverty (16).
The findings of this study are important for several reasons. To our knowledge, this is the first clinical trial to demonstrate a high rate of SVR among patients of PCPs and NPs providing independent HCV care using DAAs. The high cure rate achieved by nonspecialist providers was maintained even in patients with HIV co-infection, cirrhosis, or previous interferon experience. No baseline or clinical characteristics were associated with SVR, supporting the generalized efficacy of DAA therapy reported in other real-world cohorts (17, 18). Our study was conducted within the setting and time limitations of standard medical practice, without the use of patient navigators, in FQHCs serving an urban, socioeconomically challenged population, thus demonstrating the feasibility of delivering DAA treatment in these existing sites. Treatment was safe, with no deaths related to study participation and with an AE profile similar to that of previous LDV-SOF studies. Finally, this system of care was implemented after a 3-hour guideline-based training session. Taken together, these results support decentralization of HCV treatment to facilitate greater therapeutic capacity for the many patients who remain untreated.
Currently in the United States, only 50% of patients with HCV infection are aware of their diagnosis (19), and given the national recommendations for expanded screening (20, 21), as well as consensus guidance (22) for treatment in all but those with a short life expectancy, therapeutic demand likely will continue to increase. Models of care that rapidly increase treatment access might benefit a health care system strained by a paucity of specialists. Although ASCEND included a small number of providers limited to 2 clinic systems, the results suggest that in the DAA era, nonspecialist providers can be trained rapidly to offer a single-step "diagnosis-linkage-treatment" continuum, avoiding the need for referrals in uncomplicated cases.
Furthermore, with the exception of HIV-HCV co-infection, the baseline characteristics of patients receiving treatment from the 3 provider types were similar. No evidence was found that the relationship between provider type and SVR differed by HIV or cirrhosis status, supporting the high efficacy of nonspecialists in providing treatment to previously challenging subpopulations of the HCV epidemic.
Treatment duration was determined solely by the treating provider, and 90% of patients were assigned to receive 12 weeks of therapy. However, on the basis of the LDV-SOF labeling criteria, 56.8% of these patients (306 of 539) were eligible for 8-week therapy. Despite inclusion of 8-week labeling criteria in the training intervention, provider sentiment swayed toward longer treatment. Given the potential cost savings of a shorter treatment period, further education may be required to see this in practice.
Among 551 patients with available prescription adherence data, the vast majority (87%) picked up all prescribed medication. Although SVR is not a direct measure of medication adherence, our analysis found that patients with 100% prescription adherence had a higher SVR rate than those with less than 100% adherence, despite moderate rates of attendance to provider visits. These findings support the generalized efficacy of DAA therapy and suggest that most patients with uncomplicated HCV infection who take their medication will achieve cure, regardless of provider type.
Finally, 7.5% of patients were LTFU during the study, an attrition rate consistent with that of other real-world investigations and reflective of the complex socioeconomic dynamics of this urban cohort. Most of these patients were lost after completing treatment, with SVR data unknown. Together with adherence data, these findings support identification of patients most at risk for nonadherence, as well as counseling regarding the meaning of SVR and the importance of testing for it. A recent study of a validated assessment tool (23) suggests that such processes need not be time or resource intensive.
ASCEND had several strengths, foremost its inclusion criteria, which paralleled the LDV-SOF label. As such, the study population is generalizable to persons living with HCV infection in U.S. cities, where the prevalence of comorbid conditions, including substance use disorder and polypharmacy, is substantial. To our knowledge, the ASCEND cohort is the largest single study to date of LDV-SOF therapy in black patients.
ASCEND also had several limitations. First, providers chose patients to refer for screening. Although inclusion based on provider recommendation potentially resulted in referral bias (for example, toward adherent patients or those with greater perceived acuity), we believe this is an accurate reflection of clinical decision making by providers on a regular basis. Providers often choose to initiate treatment in patients whom they feel will be highly motivated or require medical priority for treatment. In addition, patients were not randomly assigned to the 3 types of providers. However, the distribution criteria used were chosen to mirror how patients are generally assigned to providers in clinic settings, including preserving existing patient-provider relationships whenever possible. Next, the single drug used in this study has limited variation in dosing and few common drug-drug interactions. It is unclear whether decentralized care would be as efficacious in patients receiving regimens with complex dosing variations based on genotype or resistance-associated variants or regimens including ribavirin. Finally, the primary outcome analysis was limited to ascertaining SVR and did not address other vital aspects of HCV care, including long-term surveillance for hepatocellular carcinoma or reinfection.
Of importance, this investigation differs from real-life practice in that all providers dispensed medication directly to patients at the treating facility, avoiding the rigorous process of prior authorization-including restrictions regarding provider type-currently required by public and private insurance plans and managed care organizations in most states. Because of these requirements, most PCPs and NPs in the United States currently are not permitted to provide independent HCV treatment. The ASCEND investigation suggests that such provider restrictions are not supported by evidence and stand as unnecessary hurdles in the HCV care continuum. Reversal of such policies might allow rapid escalation of safe, effective therapy for HCV infection and improve the care of patients living with this potentially fatal disease.
|
|
| |
| |
|
|
|