| |
Another statin option in HIV - Comment
|
| |
| |
Download the PDF here
Download the PDF here
In The Lancet HIV, Judith Aberg and colleagues present results of the INTREPID study.1 This study was a multicentre, double-blind, double-dummy, randomised clinical trial comparing the safety and lipid lowering effects of pitavastatin (4 mg daily) and pravastatin (40 mg daily) in 252 people with HIV and dyslipidaemia. Study participants were on suppressive antiretroviral therapy (ART), more than 80% were white men, and those with glucose intolerance, established diabetes, or coronary artery disease were not eligible to participate.
At 12 weeks, LDL cholesterol decreased more with pitavastatin than with pravastatin and these effects were sustained at 52 weeks. The proportion of adverse events were similar between the two treatment groups and no effect on fasting glucose, insulin, or glycosylated haemoglobin was recorded. This finding is important given the increased risk of diabetes with statins, particularly with high-dose statin therapy2 and with rosuvastatin.3 HIV clinicians will welcome the results of the INTREPID trial because they establish pitavastatin as an additional appropriate option to treat dyslipidemia also in HIV.
Because pitavastatin is considered to be a weaker LDL-lowering drug than atorvastatin and rosuvastatin,4 we are reassured by the efficacy of pitavastatin in INTREPID (median LDL cholesterol decrease of 31·1%). However, pitavastatin fared better in a randomised, placebo-controlled trial5 that the authors previously did in people without HIV (median LDL cholesterol concentration decrease of 38·1%). Statins are believed to be less effective at lowering LDL cholesterol concentrations in people with HIV than in the general population because of drug interactions or concurrent use of antiretroviral drugs that promote dyslipidaemia. This notion was confirmed in a large retrospective analysis6 of 550 people with HIV and 5170 seronegative patients starting statin therapy but the difference was relatively small (-25·6% adjusted LDL cholesterol change in people with HIV versus -28·3% in seronegative people).
Among statins, pitavastatin and pravastatin have the advantage of few drug interactions with ART because they are minimally metabolised by hepatic cytochrome P450 enzymes. More than 20% of INTREPID participants were treated with protease inhibitors that are no longer among preferred drugs because of their association with atherogenic dyslipidaemia, according to European and US ART guidelines. In some of these patients, changing the protease inhibitor to a drug that causes fewer metabolic disturbances could have made a statin unnecessary. Moreover, ritonavir-boosted protease inhibitors have a high interaction potential with statins. With the increasing use of integrase inhibitors with few drug interactions in recent years, such as dolutegravir and raltegravir, the clinical need for statins with low drug interaction potential has become less urgent. Nonetheless, pitavastatin might be a valuable, alternative statin choice in patients with dyslipidaemia treated with integrase inhibitors who are unable to tolerate other statins.
Is it a problem that the INTREPID study was fully funded, implemented, and analysed by the manufacturers of pitavastatin? We believe not; the authors appropriately disclose this information and the study was well conducted. However, the funders are likely to explain why pravastatin was selected as comparator. Pravastatin is a relatively weak statin,4 and, of particular interest to HIV, pravastatin might not5 (but atorvastatin might7, 8) reduce markers of chronic T-cell activation in people with HIV.
What is next? The effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in INTREPID have now been published.9 At week 52 and compared with pravastatin, pitavastatin led to somewhat larger reductions in some biomarkers (soluble CD14, oxidised LDL, and lipoprotein-associated phospholipase A2 [Lp-PLA2]) but no differences in other biomarkers that are also of interest to researchers in the field (soluble CD163, interleukin 6, monocyte chemoattractant protein-1). Also, in a study10 done in HIV-negative people, pitavastatin showed no effect (at a 2 mg daily dose) on Lp-PLA2.
Importantly, we do not yet know whether statin treatment translates into meaningful clinical benefits in people with HIV. Statin therapy reduces atherosclerotic plaque in people with HIV11 but conclusive data that statins reduce the cardiovascular event rate in this population are not yet available.12, 13 This finding is perhaps surprising given the substantial attention to the notion of accelerated atherosclerosis, or even accelerated aging, in people with HIV in the past few years.
Hopes are therefore high that the ongoing REPRIEVE trial (NCT02344290), sponsored by the manufacturer of pitavastatin and the US National Heart Lung and Blood Institute, will answer this question.14 REPRIEVE investigators intend to randomise 6500 people with HIV aged 40-75 years without known cardiovascular disease to pitavastatin 4 mg per day versus placebo and assess the cardiovascular event rate during 6 years of follow-up. Meanwhile, that lipid-lowering treatment with statins is as relevant to people with HIV at increased cardiovascular risk as it is in the general population remains a reasonable but unproved assumption.
-----------------------------------
Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial
HIV Lancet April 27 2017
Judith A Aberg, Craig A Sponseller, Douglas J Ward, Vladimir A Kryzhanovski, Stuart E Campbell, Melanie A Thompson
Summary
Background
People living with HIV-1 infection are at greater risk for cardiovascular disease than seronegative adults. Treatment of dyslipidaemia with statins has been challenging in people with HIV because of an increased potential for drug interactions due to competing cytochrome P450 metabolism between statins and commonly used antiretroviral agents. Neither pitavastatin nor pravastatin depend on cytochrome P450 for primary metabolism. We aimed to assess the safety and efficacy of pitavastatin versus pravastatin in adults with HIV and dyslipidaemia.
Methods
In the INTREPID (HIV-infected patieNts and TREatment with PItavastatin vs pravastatin for Dyslipidemia) randomised, double-blind, active-controlled, phase 4 trial (INTREPID, we recruited adults aged 18-70 years with controlled HIV (with CD4 counts >200 cells per μL and HIV-1 RNA <200 copies per mL) on antiretroviral therapy for at least 6 months and dyslipidaemia (LDL cholesterol 3·4-5·7 mmol/L and triglycerides ≤4·5 mmol/L) from 45 sites in the USA and Puerto Rico. Patients being treated with darunavir, or who had homozygous familial hypercholesterolaemia or any condition causing secondary dyslipidaemia, or a history of statin intolerance, diabetes, or coronary artery disease were not eligible. We randomly assigned patients (1:1) to pitavastatin 4 mg or pravastatin 40 mg with matching placebos once daily orally for 12 weeks, followed by a 40 week safety extension. Randomisation was stratified by viral hepatitis B or C coinfection and computer-generated. Investigators, patients, study staff, and those assessing outcomes were masked to treatment group. The primary endpoint was percentage change in fasting serum LDL cholesterol from baseline to week 12 and the primary efficacy analysis was done in the modified intention-to-treat population. The safety analysis included all patients who took at least one dose of study medication. This study is registered with ClinicalTrials.gov, number NCT01301066.
Findings
Between Feb 23, 2011, and March 29, 2013, we randomly assigned 252 patients to the pitavastatin (n=126) or pravastatin group (n=126). LDL cholesterol reduction was 31·1% with pitavastatin and 20·9% with pravastatin (least squares mean difference -9·8%, 95% CI -13·8 to -5·9; p<0·0001) at 12 weeks. At week 52, four patients (3%) in the pitavastatin group and six (5%) in the pravastatin group had virological failure, with no significant difference between treatments. Both treatments had neutral effects on glucose metabolism parameters. 85 patients treated with pitavastatin (68%) and 88 patients treated with pravastatin (70%) reported treatment-emergent adverse events, and these caused study discontinuation in six patients (5%) versus five patients (4%). No serious adverse event occurred in more than one participant and none were treatment-related according to investigator assessment. The most common treatment-emergent adverse events were diarrhoea in the pitavastatin group (n=12, 10%) and upper respiratory tract infection in the pravastatin group (n=14, 11%). 11 treatment-emergent serious adverse events were noted in seven patients (6%) in the pitavastatin group (atrial septal defect, chronic obstructive pulmonary disease, chest pain, diverticulitis, enterovesical fistula, gastroenteritis, viral gastroenteritis, herpes dermatitis, multiple fractures, respiratory failure, and transient ischaemic attack) and four events in three patients (2%) in the pravastatin group (cerebrovascular accident, arteriosclerosis coronary artery, myocardial infraction, and muscle haemorrhage). In the pravastatin treatment group, one additional patient discontinued due to an adverse event (prostate cancer that was diagnosed during the screening period, 42 days before first dose of study treatment, and therefore was not a treatment-emergent adverse event).
Interpretation
The INTREPID results support guideline recommendations for pitavastatin as a preferred drug in the treatment of dyslipidaemia in people with HIV.
Introduction
Antiretroviral therapy (ART) has had a major effect on the survival of people living with HIV, with some studies now estimating that the lifespan of people who achieve viral suppression might approximate that of the general population.1, 2 However, even with complete viral suppression, there is evidence of increased immune activation and resultant residual inflammation contributing to additional non-AIDs-related conditions, including cardiovascular disease.3, 4 The risk for myocardial infarction is 1·5-2·0 times higher in people with HIV than in those who are uninfected,5, 6 and dyslipidaemia has been reported in up to 80% of individuals with HIV.7 Increases in lipid concentrations associated with specific ARTs, notably the protease inhibitors, are less commonly observed with newer medications.8 Neither ageing alone nor traditional cardiovascular disease risk factors fully explain the excess cardiovascular risk noted in people with HIV. Possible mechanisms working separately or in combination include chronic immune activation and inflammation caused by the HIV infection itself as well as associated senescence and dysregulation of the immune system.9 A unique pathophysiology of atherosclerosis in the setting of HIV highlights the need for tailored primary cardiovascular disease prevention strategies in this population. Regardless of the biological cause of dyslipidaemia, the challenge is how best to manage this disorder in people living with HIV.
Research in context
Evidence before this study
We searched PubMed and the Cochrane Library from Feb 1, 1991, to Feb 1, 2011, before starting the study and Feb 1, 2011, through to Jan 23, 2017, in preparation for this report, without language restrictions, with the search terms "HIV" AND "statin" ("HMG-CoA" OR "hydroxymethylglutaryl-CoA reductase inhibitors" OR "statin"), "HIV" AND "statin" ("HMG-CoA" OR "hydroxymethylglutaryl-coenzyme A reductase inhibitors" OR "statin") AND "HAART" OR "ART" OR "protease inhibitor", and "HIV" AND "dyslipidemia" ("dyslipidemias" OR "dyslipidemia") AND "prospective randomized controlled trial" ("RCT" or "prospective* randomized* controlled* trial*") AND "HAART" OR "ART" OR "protease inhibitor." We identified eight prospective, randomised, controlled trials that evaluated statins in adults with HIV. Three were placebo-controlled, double-blind studies and the other five were open-label studies. In the placebo-controlled studies, the sample sizes ranged from 21 to 33 patients; study periods ranged from 8 weeks to 12 weeks; all studies assessed pravastatin; and the pravastatin LDL cholesterol reductions ranged from -19% to -24%. In the five open-label studies, the sample sizes ranged from 74 to 174 patients, and the study periods ranged from 45 days to 12 months. In the two open-label studies that were 12 months in duration, LDL cholesterol reductions ranged from -18% to -26% with pravastatin, fluvastatin, atorvastatin, or rosuvastatin. We also identified nine studies that were non-randomised or retrospective in overall design.
Added value of this study
The INTREPID trial is, to our knowledge, the first randomised, double-blind, active-controlled, head-to-head trial assessing the efficacy and safety of statin therapy in adults with HIV and dyslipidaemia taking antiretroviral therapy (ART). Moreover, we believe that this trial is the first to evaluate the efficacy and safety of pitavastatin in this difficult-to-treat population. Our data show that pitavastatin 4 mg lowers LDL cholesterol and maintains moderate intensity LDL cholesterol reduction (as defined by the 2013 American College of Cardiology and American Heart Association guideline on the treatment of blood cholesterol) more than pravastatin 40 mg. Additionally, because its metabolism is not cytochrome P450 dependent, pitavastatin can be used in the setting of complex background ART. Importantly, with respect to safety, there were no significant changes in the variables of insulin resistance for either treatment at week 12 or week 52 in this population, which is at greater risk for incident diabetes. This finding is further evidence that statins differ in their extent of risk for such a side-effect of statin therapy. Taken together, pitavastatin might be an optimum treatment option for adults with HIV-associated dyslipidaemia who have a high risk of cardiovascular disease, and subsequently the preliminary results in abstracts from the INTREPID study are cited in the 2015 National Lipid Association Part 2 recommendations that pitavastatin is a preferred statin in the treatment of dyslipidaemia for people living with HIV.
Implications of all the available evidence
People living with HIV are now reaching advanced ages similar to adults without the virus, but they have an increased risk of cardiovascular disease due to chronic inflammation and immune activation among other reasons. This population has the additional concerns of complicated drug interactions with lipid-modifying agents combined with ART and the increased risk for insulin resistance and incident diabetes. The INTREPID study identifies potent treatment options for HIV-associated dyslipidaemia with a favourable benefit-to-risk profile. The REPRIEVE trial (NCT02344290) is investigating the effect of statin therapy (pitavastatin) for the primary prevention of cardiovascular disease in adults with HIV who do not have a clinical indication for taking statins.
With few exceptions, statin therapy is the recommended first-line treatment for dyslipidaemia,10, 11 although the effect can be attenuated in people with HIV,12 possibly because of the factors mentioned previously. The use of statins in the setting of ART has been challenging because of drug interactions, which are sometimes unpredictable, leading to intolerance or reduced efficacy. Protease inhibitors, non-nucleoside reverse transcriptase inhibitors, and most statins are metabolised via the cytochrome P450 (CYP) enzyme system.8 Simvastatin and lovastatin are contraindicated with protease inhibitors, atorvastatin doses should not exceed 20 mg/day if given with protease inhibitors, and rosuvastatin requires a dose reduction with selected protease inhibitors.8 Pitavastatin is primarily metabolised by glucuronidation with only marginal metabolism by CYP2C9 and to a lesser extent CYP2C8,8, 13 and there are no contraindications or dose limitations with pitavastatin and protease inhibitor therapy.8, 13, 14 Pravastatin is also not dependent on CYP metabolism.8 Pitavastatin 4 mg and pravastatin 40 mg are both classified as moderate-intensity statins and share similar potency.10, 11 At the time of this study, pravastatin was a commonly used drug in the management of dyslipidaemia in people living with HIV, on the basis of drug interaction data in the absence of randomised, double-blind comparative trials of statins in this population.14, 15
Previously, pitavastatin 4 mg was compared with pravastatin 40 mg in a randomised, active-controlled, non-inferiority phase 3 study16 in elderly adults (≥65 years) without HIV with primary hyperlipidaemia or mixed dyslipidaemia and showed LDL cholesterol concentration was reduced more significantly with pitavastatin 4 mg compared with pitavastatin 40 mg after 12 weeks of treatment.
We aimed to assess the safety and effectiveness of pitavastatin 4 mg compared with pravastatin 40 mg on LDL cholesterol and other lipid variables in adults with HIV and dyslipidaemia who were also on ART.
Methods
Study design
INTREPID (HIV-infected patieNts and TREatment with PItavastatin vs pravastatin for Dyslipidemia) was a randomised, double-blind, double-dummy, active-controlled, phase 4 superiority trial that compared the effect of pitavastatin 4 mg versus pravastatin 40 mg on LDL cholesterol reduction in adults with HIV and dyslipidaemia over 12 weeks, followed by a 40 week safety extension period. In addition to its evaluation in the non-HIV infected population,16 we also selected the maximum daily dose of 4 mg for pitavastatin on the basis of its minimal effect on lopinavir and ritonavir exposures in a phase 4 clinical pharmacokinetic study.17 In that same study, non-significant changes were shown for the pharmacokinetics and overall systemic exposures of pitavastatin.17Regarding the active control, we selected the 40 mg dose of pravastatin because it is the starting dose recommended and approved by the US Food and Drug Administration and pravastatin was a recommended statin therapy for dyslipidaemia in individuals with HIV.14
Dosing was once daily orally in the morning with or without food. There were 42 study sites in the USA and three in Puerto Rico. Institutional review boards at all participating sites approved the study.
Patients
Patients meeting the initial screening criteria had a 4 week dietary stabilisation period. Study investigators instructed them to follow a fat and cholesterol restrictive diet during this period and continue this diet throughout the study.13 Although lifestyle counselling is a key element of preventive efforts at all levels of risk, the purpose of these dietary restrictions was also to minimise fluctuations in lipid profiles resulting from a wide range of dietary habits across a population of patients and to allow for the most accurate baseline lipid profile and comparison between measurements at randomisation and during follow-up. We required a medication washout period of at least 4 weeks for those taking statins or other agents not permitted in the study.
To be eligible for the study, the protocol required patients aged 18-70 years to have been on ART for at least 6 months before randomisation, and this therapy must have been stable in the 3 months before randomisation with no anticipated need to change during the first 12 weeks of the study. Also, for at least 3 months before randomisation, patients had to have CD4 counts of more than 200 cells per μL and HIV-1 RNA of less than 200 copies per mL. After completion of the dietary stabilisation and statin washout period, patients had to have documented dyslipidaemia, defined as fasting serum LDL cholesterol 3·4-5·7 mmol/L (130-220 mg/dL) and triglycerides 4·5 mmol/L or less (≤400 mg/dL). Use of darunavir was not permitted on the basis of a reported 81% increase in pravastatin area under the curve (total drug exposure) and the recommendation to use the lowest necessary dose of pravastatin with darunavir administration.18 Patients with homozygous familial hypercholesterolaemia or any condition causing secondary dyslipidaemia, or history of statin intolerance, diabetes (or screening fasting serum glucose >6·9 mmol/L), or coronary artery disease were not eligible for study enrolment. All patients provided written informed consent before the start of any study procedures.
Randomisation and masking
Randomisation was done after completion of the dietary stabilisation and washout period for those patients who continued to meet eligibility criteria. We randomly assigned patients (1:1) with an interactive voice response system and the randomisation schedule was prepared by the contract research organisation's biostatistics department. Randomisation was stratified by presence or absence of hepatitis B or C co-infection. Study sites instructed all patients to take one tablet and one capsule per day from bottles supplied in each unique treatment kit. The placebo tablet and capsule were identical in appearance to their respective active product. Patients assigned to pitavastatin received one tablet of pitavastatin 4 mg and one placebo capsule. Patients assigned to pravastatin received one capsule of pravastatin 40 mg (two tablets of pravastatin 20 mg over encapsulated) and one placebo tablet. We locked the database after the last participant completed the 12 week efficacy portion of the trial. An unblinded team that functioned independently of the blinded study team analysed the data at 12 weeks. The study patients, investigators, site staff, and the blinded study team remained blinded to all study data throughout the study including completion of the 40 week extension. No unblinding occurred during the study.
Procedures
We assessed patients every 4 weeks through to week 12 of the study, and then again at weeks 24, 36, 48, and 52. Samples for efficacy and safety assessments were obtained after an overnight fast of at least 8 h. On clinic visit days, patients took their study medication after the blood draw, and sites assessed adverse events, concomitant drugs, drug adherence, and obtained clinical laboratory samples. We used COBAS AmpliPrep and COBAS TaqMan HIV-1 Test, version 2.0 (Roche Molecular Diagnostics, Pleasanton, CA, USA) to quantify HIV-1 RNA in plasma and reported viral loads of less than 20 copies per mL as zero for the purpose of analysis.
Outcomes
The primary efficacy endpoint was the percentage change in fasting serum LDL cholesterol concentrations from baseline to week 12. We assessed the following secondary efficacy variables through to week 52: LDL cholesterol, HDL cholesterol, total cholesterol, triglycerides, total cholesterol to HDL cholesterol ratio, non-HDL cholesterol, non-HDL cholesterol to HDL cholesterol ratio, apolipoprotein A1, apolipoprotein B, apolipoprotein B to apolipoprotein A1 ratio, oxidised LDL, high-sensitivity C-reactive protein, and LDL cholesterol target attainment. We established a target LDL cholesterol value for each participant based on Framingham risk calculation (National Cholesterol Education Program Adult Treatment Panel III19) for coronary heart disease at screening, and assessed each participant's ability to achieve the target LDL cholesterol value at weeks 12 and 52.
Assessment of the effect of ritonavir-based therapy on the primary endpoint was a prespecified exploratory endpoint. A post-hoc analysis assessed the effect of concomitant efavirenz treatment on LDL cholesterol reduction. In addition to the proportion of patients with adverse events, prespecified safety endpoints also included the changes from baseline in CD4 cell counts, HIV-1 RNA, fasting glucose, and glycated haemoglobin (HbA1c). Determination of change in fasting plasma insulin concentrations was also prespecified but considered an exploratory endpoint and was analysed with the modified intention-to-treat population. Post-hoc analyses with the modified intention-to-treat population evaluated changes from baseline in the homoeostasis model assessment-estimated insulin resistance (HOMA-IR) index and the quantitative insulin-sensitivity check index (QUICKI).
Statistical analysis
We used Statistical Analysis System (version 9.1 or higher) for all analyses. Results are expressed as a number and percentage of patients for categorical variables and mean, median, SD, and minimum and maximum for continuous variables. All statistical tests were two sided with a significance level of 0·05, unless otherwise specified. We designed the study to have 90% power to detect a reduction in LDL cholesterol of 42% (SD 20) for pitavastatin versus 34% (14) for pravastatin, with a two-sided p value of 0·05. Allowing for a 10% discontinuation rate, we estimated the final sample size to be about 250 patients (125 per treatment group).
For the primary efficacy analysis, we used the modified intention-to-treat population (defined as all randomly assigned patients who received at least one dose of study medication and had at least one lipid assessment after baseline) using an ANCOVA model, with a percentage change in LDL cholesterol from baseline to week 12 as the dependent variable and treatment as the independent variable, after adjusting for site and viral hepatitis B or C infection status at randomisation. If the week 12 measurement was missing, we carried forward the last scheduled post-baseline measurement before week 12 as the endpoint measurement (last observation carried forward method). The ANCOVA estimates included least squares means, SEs, two-tailed 95% CIs, and p values of the percentage change within each treatment group and for the comparisons between pitavastatin and pravastatin. We tested the residuals from the ANCOVA model for normality. If the assumption of normality was rejected, we also analysed the results using the van Elteren test to assess the treatment difference.20Analysis of the effect of concomitant ritonavir use (prespecified) and efavirenz use (post hoc) on LDL cholesterol was identical to the primary efficacy analysis but with an additional adjustment made for concomitant ritonavir or efavirenz use.
We applied the previous methods described for the primary efficacy analysis to the analysis of the secondary efficacy variables. The exception was LDL cholesterol target attainment, which used a Cochran-Mantel-Haenszel test (odds ratios [OR], 95% CIs, and p values) to compare pitavastatin and pravastatin, adjusting for viral hepatitis B or C infection status at randomisation. We used the safety population, defined as all randomised patients who took at least one dose of study medication, for all safety analyses, and summarised the safety data by treatment group with descriptive statistics. The Medical Dictionary for Regulatory Activities was used to code all adverse events.
As an amendment to the statistical analysis plan, we did supportive analyses that did not rely on the last observation carried forward method. We used the modified intention-to-treat population and applied a mixed-effects model repeated measures analysis to the primary endpoint and selected key secondary endpoints (LDL cholesterol at week 52 and apolipoprotein B, total cholesterol, triglycerides, HDL cholesterol, and non-HDL cholesterol at weeks 12 and 52). To address the potential effect of missing data for the randomised population, we implemented the multiple imputation method, with the same ANCOVA model specified for the primary analysis. We applied this technique to the primary efficacy endpoint and the previously mentioned selected key secondary endpoints for consistency and sensitivity in reporting results compared with the method of mixed-effects model repeated measures. The trial is registered with ClinicalTrials.gov, number NCT01301066.
Role of the funding source
Kowa Pharmaceuticals America and Eli Lilly and Company funded the study in full. The funders had a role in the study design, data collection, data analysis and interpretation, and writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Between Feb 23, 2011, and March 29, 2013, we screened 594 patients (figure 1). Failure to meet at least one of the inclusion criteria accounted for most of the excluded patients. The two most common reasons were not meeting LDL cholesterol eligibility criteria after the statin washout or dietary lead-in period and abnormal screening laboratory values. We randomly assigned 126 patients to pitavastatin 4 mg and 126 to pravastatin 40 mg (figure 1). Mean age was 50 years and 217 (86%) of 252 patients were men (table 1). The mean duration of HIV infection was 12·6 years (SD 7·5 years), and hepatitis B or C virus was present in about 10% of patients at randomisation. 92% of patients reported taking nucleoside reverse transcriptase inhibitors, 54% non-nucleoside reverse transcriptase inhibitors, and 40% protease inhibitors (appendix p 1). A total of 224 patients completed 12 weeks of the study and 190 completed all 52 weeks (figure 1). 27 (21%) of 126 patients treated with pitavastatin and 35 (28%) of 126 patients treated with pravastatin discontinued early from the study. Discontinuations because of adverse events occurred in 12 patients, six in each treatment group.
The reduction in LDL cholesterol concentrations was significantly greater in the pitavastatin 4 mg group (31·1%) than in the pravastatin 40 mg group (20·9%) at 12 weeks of therapy: treatment difference -9·8 (95% CI -13·8 to -5·9; figure 2A), and the benefit was sustained at week 52. At week 52, the least squares mean treatment difference was -8·4% (95% CI -13·1 to -3·6; figure 2A) The differences between treatments remained significant after adjusting for site, hepatitis B or C infection, and either efavirenz or ritonavir use (appendix p 2).
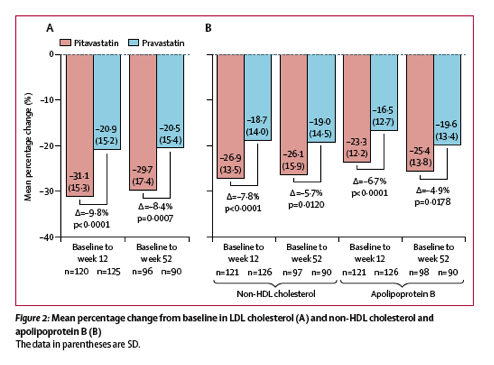
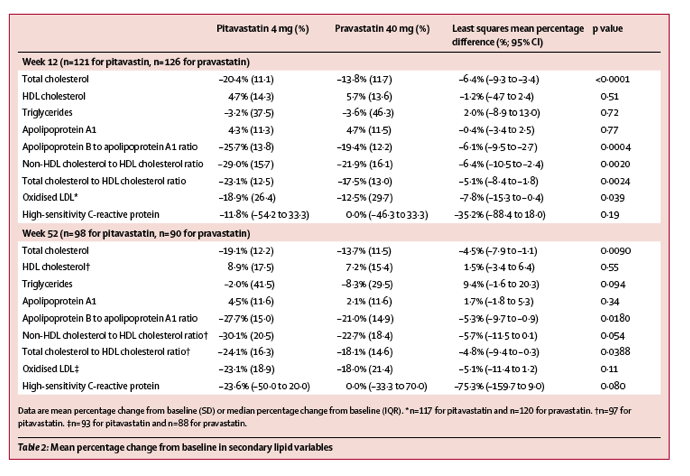
After weeks 12 and 52 of therapy, the reductions in non-HDL cholesterol and apolipoprotein B were significantly greater with pitavastatin than with pravastatin (figure 2B). Additionally, the differences between treatments were also in favour of pitavastatin for total cholesterol, apolipoprotein B to apolipoprotein A1 ratio, and total cholesterol to HDL cholesterol ratio (table 2). Pitavastatin reduced non-HDL cholesterol to HDL cholesterol ratio and oxidised LDL significantly more than pravastatin at week 12, but the differences between treatments were not sustained through to week 52 (table 2). There were no differences between treatment groups in changes in apolipoprotein A1, triglycerides, HDL cholesterol, or high-sensitivity C-reactive protein levels at either week 12 or week 52 (table 2).
Patients in the pitavastatin group were more likely to reach their LDL cholesterol target at weeks 12 and 52 than those receiving pravastatin. At week 12, 104 (87%) of 120 patients in the pitavastatin group reached their LDL cholesterol target compared with 83 (66%) of 125 patients in the pravastatin group (3·3, 1·7-6·3; p=0·0002). One patient in each group did not have a valid LDL cholesterol test and therefore were not evaluable. Similar findings occurred at week 52: 79 (82%) of 96 compared with 59 (66%) of 90 (OR 2·4, 95% CI 1·2-4·6; p=0·0123). Two patients in the pitavastatin group were not evaluable for LDL cholesterol at week 52 in the modified intention-to-treat population.
Results from the supportive analyses at weeks 12 and 52 with the mixed-effects model repeated measures and multiple imputation methods were consistent with the results with the last observation carried forward method. The mixed-effects model repeated measures included all patients in the modified intention-to-treat population who had at least one non-missing scheduled assessment after baseline. A total of 17 patients who were randomly assigned and treated were not included in this analysis. Of these, five patients received pitavastatin but had no on-treatment lipid assessment, so were excluded from the modified intention-to-treat population. The additional 12 patients not included in the mixed-effects model repeated measures analysis were included in the modified intention-to-treat population: they had a non-missing, post-baseline value, but not at scheduled visits. The multiple imputation analysis included all randomly assigned patients. When the mean values for the primary efficacy variable were reviewed by reason of study discontinuation, we noted that these values for patients who discontinued the study supported those who completed the study.
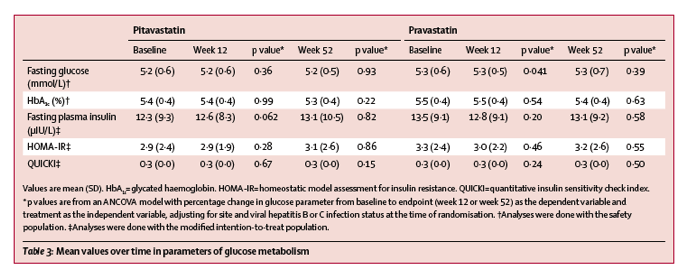
Compared with baseline, we noted no significant effects on fasting glucose or HbA1c for either pitavastatin or pravastatin at 52 weeks of treatment (table 3), and no patient had a post-baseline abnormal fasting glucose or HbA1c level that was reported as a treatment-emergent adverse event. The changes in fasting plasma insulin, HOMA-IR, and QUICKI also were not significantly different from baseline for either treatment.
There were no significant between-treatment differences in CD4 cell counts or HIV-1 RNA at week 52 (appendix p 1). Through 52 weeks of therapy, virological failure (defined as HIV-1 RNA >200 copies per mL and >2 copies per mL increase from baseline) occurred in four (3%) of 126 patients in the pitavastatin group and six (5%) of 126 patients in the pravastatin group, with no significant differences between treatments (table 4). Increases in HIV-1 RNA loads were associated with treatment-emergent adverse events in two (2%) of 126 patients in the pitavastatin group and three (2%) of 126 patients in the pravastatin group, but none were considered related to study treatment. There was one patient with a decrease in CD4 cell count in the pitavastatin treatment group that was reported as a treatment-emergent adverse event, but the investigator assessed the event as not related to study treatment.
The reductions in LDL cholesterol were similar in users and non-users of efavirenz within treatment groups. Patients receiving efavirenz had reductions in LDL cholesterol concentrations of 32·0% at week 12 (45 of 121 patients) and 31·0% at week 52 (35 of 121 patients) in the pitavastatin group and 18·6% at week 12 (49 of 126 patients) and 19·2% at week 52 (36 of 126 patients) in the pravastatin group. For patients who did not receive efavirenz, the week 12 reduction was 30·5% and 28·9% at week 52 for the pitavastatin group, and 22·4% at week 12 and 21·3% at week 52 in the pravastatin group. Likewise, the reduction in LDL cholesterol concentration was also not affected by the concomitant use of ritonavir in either treatment group (week 12, 38 of 121 patients in the pitavastatin group and 45 of 126 patients in the pravastatin group on ritonavir vs 82 patients in the pitavastatin group and 80 patients in the pravastatin group not on ritonavir; week 52, 34 of 121 patients on pitavastatin and 33 of 126 patients on pravastatin on ritonavir vs 62 patients on pitavastatin and 57 patients on pitavastatin not on ritonavir). At week 52, the reduction in LDL cholesterol concentration was 26·6% with and 31·4% without concomitant ritonavir in the pitavastatin group and 18·3% with and 21·8% without in the pravastatin group. However, sample sizes in these subgroup analyses were small, and the study was not powered adequately to detect small between-treatment differences
Treatment-emergent adverse events occurred in 85 (68%) of 126 patients treated with pitavastatin and in 88 (70%) of 126 patients treated with pravastatin. Most of these events were of mild (60% overall) or moderate (29% overall) severity. The most common treatment-emergent adverse events were 12 (10%) patients with diarrhoea in the pitavastatin group and 14 (11%) patients with an upper respiratory tract infection in the pravastatin group. Myalgia occurred in two (2%) patients in the pitavastatin group and three (2%) patients in the pravastatin group, and caused study discontinuation in one (1%) patient in each treatment group. Treatment-emergent adverse events occurred in 16 (13%) of 126 pitavastatin-treated patients and 12 (10%) of 126 patients treated with pravastatin. Of the treatment-related events, no event occurred in more than two patients in the pitavastatin group or more than three patients in the pravastatin group. 11
treatment-emergent serious adverse events were noted in seven (6%) of 126 patients in the pitavastatin group (atrial septal defect, chronic obstructive pulmonary disease, chest pain, diverticulitis, enterovesical fistula, gastroenteritis, viral gastroenteritis, herpes dermatitis, multiple fractures, respiratory failure, and transient ischaemic attack) and four events in three (2%) patients in the pravastatin group (cerebrovascular accident, arteriosclerosis coronary artery, myocardial infraction, and muscle haemorrhage). No serious adverse event occurred in more than one patient, and no serious adverse event was treatment-related according to investigator assessment. One serious adverse event (ischaemic cerebrovascular event in the pravastatin group) resulted in study discontinuation. There were no deaths in the study.
Discussion
In the INTREPID study, pitavastatin was superior to pravastatin in reducing LDL cholesterol (8-10% treatment difference) in patients with HIV, which is similar to findings in previous comparisons in the general population with dyslipidaemia.16, 21 Pitavastatin also showed greater improvements in atherogenic lipid or lipoprotein variables, including non-HDL cholesterol and apolipoprotein B, whereas there were no differences between treatments in changes in triglycerides or HDL cholesterol. The lipid improvements noted after 12 weeks of therapy were sustained up to 52 weeks, and the sensitivity and supportive analyses confirmed these findings. Patients in both treatment groups had high baseline high-sensitivity C-reactive protein values of more than 2 mg/L; however, the extent of change at weeks 12 and 52 were not significant for either treatment group. These results support recommendations that high-sensitivity C-reactive protein is not useful in the determination of cardiac risk or response to statin therapy in people living with HIV.8, 10 There were no between-treatment differences in CD4 cell counts or HIV-1 RNA at week 52. Likewise the occurrence of virological failure was similar for both treatments. Furthermore, there were no clinically meaningful changes in safety between short-term and long-term treatments, and no unexpected safety concerns compared with other pitavastatin clinical trials.13, 16, 21 Less than 5% of study patients discontinued treatment because of adverse events.
Although nucleoside reverse transcriptase inhibitors do not seem to interact with statins, some non-nucleoside reverse transcriptase inhibitors, including efavirenz, have resulted in changes in statin plasma concentrations.8 Efavirenz has been shown to decrease the area under the curve of pitavastatin by 11% and increase its maximum serum concentration by 20%,22 but no pitavastatin dose adjustment was necessary.10, 13 The area under the curve of pravastatin has been shown to decrease by 40% with efavirenz,23 and dose adjustments (not to exceed the maximum recommended dose) according to lipid response is recommended.8 We did not permit statin dose adjustments in this trial because of the need for placebo. Therefore, to exclude any bias, we did a post-hoc analysis to evaluate the effect of efavirenz (either as a monotherapy or in a combination) on LDL cholesterol concentrations. Use of efavirenz did not affect the overall reduction in LDL cholesterol in patients treated with pravastatin, and the 12 and 52 week data support the significant LDL cholesterol reduction from baseline as observed in the overall study results.
Not surprisingly, due to its use as a pharmacokinetic enhancer for other ART medications, ritonavir was the most commonly used protease inhibitor in the study. Because of a shared metabolic pathway (CYP3A4), ritonavir and other protease inhibitors might increase the plasma concentration of some statins. Neither pitavastatin nor pravastatin depend on the CYP450 enzyme system for their metabolism.8, 13 No clinically significant pharmacokinetic interactions have been noted with the coadministration of pitavastatin and atazanavir,13 ritonavir-boosted lopinavir,13, 17 or ritonavir-boosted darunavir,13, 22, 24 and no dose adjustments are necessary.8, 10, 13 In the present study, the reductions in LDL cholesterol concentrations were similar in patients who were treated with ritonavir and patients who were not and reflected the overall study results.
Unlike ritonavir, the pharmaceutical enhancer cobicistat has no anti-HIV activity and no effect on CYP2C8 or CYP2C9.25 However, similar to ritonavir, cobicistat is a potent inhibitor of CYP3A4, and increases the plasma concentrations of certain ART, including the integrase inhibitor elvitegravir, and would be expected to increase the plasma concentrations of statins that depend on cytochrome P450 for their metabolism. Because pitavastatin is not dependent on the cytochrome P450 enzyme system for its metabolism, an interaction with cobicistat is not anticipated. However, this expectation will require confirmation in clinical trial settings.
Data have suggested that the diabetogenic effects of statin therapy are dependent on both the drug and dose.26, 27 This finding is an area of concern because people with HIV are at increased risk for impaired glucose metabolism.28 In individuals with HIV, rosuvastatin 10 mg was reported to increase markers of insulin resistance, particularly HOMA-IR, which showed a 78% increase from baseline at week 48 and remained elevated up to week 96.29 In the present study, we noted no significant long-term changes (at week 52) on any of the glycaemic indices for either pitavastatin or pravastatin. Blood glucose concentrations were also not significantly affected by pitavastatin in a study of patients with a known history of type 2 diabetes.30 These data for pitavastatin, along with previous studies in one meta-analysis,31 show that pitavastatin has a neutral effect on variables of glucose metabolism and incidence of new-onset diabetes.
In consideration of the strengths and limitations of the present study, an important strength is that INTREPID represents the only randomised, double-blind statin trial versus an active comparator, to our knowledge, in patients with chronic HIV-1 infection and dyslipidaemia. Moreover, this study allowed all concomitant ART except the protease inhibitor darunavir, as explained earlier, and followed up patients for up to 52 weeks to measure long-term safety and efficacy in this chronic disease population. Several limitations can also be considered. Women and African Americans were under-represented compared with the typical US population living with HIV-1. As discussed, we amended the statistical analysis plan to include supportive analyses, namely mixed-effects model repeated measures and multiple imputation techniques, rather than rely on last observation carried forward to provide the most appropriate estimate of the treatment effect. In particular, we used the assessment of the de-facto (intention-to-treat) estimate, particularly the difference in percentage change in fasting serum LDL cholesterol concentrations from baseline in all randomly assigned patients regardless of adherence to treatment or use of subsequent therapy, as well as accounting for missing data. The additional analyses fully supported the findings contained in the primary assessment of efficacy, accounting for any missing data. Secondly, in choosing pravastatin as the active comparator, darunavir was an exclusionary ART based on the known increased pharmacokinetic exposure of pravastatin when used in combination. Ideally, inclusion of all ARTs would have been advantageous to further support the absence of significant drug interactions amid complex HIV regimens. With regard to variables of glucose metabolism, although the protocol design included fasting plasma insulin as a prespecified safety exploratory measurement, we calculated the insulin resistance markers HOMA-IR and QUICKI as post-hoc assessments. Notwithstanding, the non-significant changes in these variables of glycaemic control represent invaluable information in a population at much risk for impaired glucose tolerance and incident diabetes.
In conclusion, individuals with HIV are at greater risk for cardiovascular disease than seronegative individuals, and clinicians continue to face challenges in the management of contributing factors such as dyslipidaemia and insulin resistance in this population. In this 52 week clinical trial in adults with HIV and dyslipidaemia, the reduction in the atherogenic lipid variables was significantly greater for patients treated with pitavastatin 4 mg than for patients treated with pravastatin 40 mg without adverse changes in measurements of glucose metabolism and insulin resistance. To better characterise the increased atherogenic risk noted in people with HIV, further clinical assessments are needed. The REPRIEVE trial (NCT02344290), a long-term, 6 year, primary prevention outcome trial in adults with HIV, is assessing the effect of pitavastatin 4 mg versus therapeutic lifestyle changes on HIV-specific markers of immune activation and inflammation, changes in vasculature, and the prevention of cardiovascular disease events. Data from REPRIEVE and other investigations exploring the vascular dysfunction observed in people with HIV should help clinicians to understand and to manage the growing cardiovascular disease risk in this advancing age population with a chronic disease state.
This online publication has been corrected. The corrected version first appeared at thelancet.com/hiv on April 27, 2017
Correction
Published: 27 April 2017
Aberg JA, Sponseller CA, Ward DJ, et al. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV 2017; published online April 13. http://dx.doi.org/10.1016/S2352-3018(17)30075-9-The funder's name should be "Eli Lilly and Company" in the Funding section of the Summary and in the Role of the funding source. In the Declaration of interests section and the affiliations, Vladimir A Kryzhanovski is an employee of Lilly USA, LLC. These corrections have been made as of April 27, 2017.
|
|
| |
| |
|
|
|