| |
Long Acting HIV ARTs - is This the Future - implants, nano formulations
|
| |
| |
Reported by Jules Levin. NATAP
"The abstract will provide an update on 10 patients with HIV who have successfully achieved complete viral load suppression for more than two years with PRO 140 administered in weekly subcutaneous injections."
We believe PRO 140 has demonstrated its value as a combination therapy and as a single agent in patients with the R5 strain of HIV and are hopeful that receiving Breakthrough Therapy Designation will speed our BLA process to get this product to the market."
CROI: PRO 140 Single-Agent Maintenance Therapy for HIV-1 Infection: A 2-Year Update - (03/01/17)
CROI: LONG-ACTING IBALIZUMAB IN PATIENTS WITH MULTI-DRUG RESISTANT HIV-1: A 24-WEEK STUDY - (02/16/17)
CROI: Theratechnologies Announces New Data from the Pivotal Phase III Trial of HIV Monoclonal Antibody and Long-Acting Investigational Antiretroviral Ibalizumab - (02/16/17)
Bi-weekly IBA plus OBR maintained virologic efficacy and was well tolerated through Wk 24 in patients with very limited treatment options due to resistance to approved ARV agents.
Ibalizumab has received "Breakthrough Therapy" designation from the FDA. This designation is given if a therapy may provide a substantial improvement over what is currently available to address a serious and life-threatening condition. Ibalizumab also received "Orphan Drug" designation by the FDA.
Multi-drug resistant (MDR) HIV-1 has been associated with a higher risk of disease progression and death. Antiretroviral agents (ARVs) with new mechanisms of action are necessary for patients with MDR HIV-1. The humanized monoclonal antibody, ibalizumab (IBA), is a long-acting ARV with a unique binding specificity allowing it to block viral entry into host cells. In the registrational Phase 3 study in MDR HIV-1 patients, TMB-301, IBA previously showed significant viral load (VL) reductions 7 days after initial dosing when added to a failing ARV regimen. Here, we describe the sustained efficacy, safety and tolerability of IBA through Week (wk) 24 of treatment.
TMB-301 is an open-label study investigating the antiviral activity and safety of IBA plus an optimized background regimen (OBR) in treatment-experienced patients with MDR HIV-1. Following a 7-day monitoring period of patients on a failing ARV regimen, an intravenous (IV) loading dose of 2000 mg IBA was administered (functional monotherapy). On Day 14, an OBR was added with at least one additional sensitive agent and patients continued on an IV maintenance dose of 800 mg IBA every two wks for 24 wks. Efficacy and safety endpoints were evaluated.
CROI: Antiviral Activity of EFdA [MK-8591] Against NRTI-Sensitive and -Resistant Strains of HIV-2 - (02/24/17)
CROI: MK-8591 Concentrations at Sites of HIV Transmission and Replication - (02/23/17)
CROI: Discovery of Novel Potent HIV Capsid Inhibitors with Long-Acting Potential- (02/16/17)
CROI: Long Acting HIV ART - is this the future? Nanoformulations - Implants - (03/02/17)
Long-Acting Antiretroviral Therapy: A Shot in the Dark or a Paradigm Shift?
Charles W Flexner
Johns Hopkins University, Baltimore, MD, USA
http://www.croiwebcasts.org/console/player/33659?mediaType=slideVideo&
CROI:A LONG-ACTING NANOFORMULATED CABOTEGRAVIR PRODRUG FOR IMPROVED ANTIRETROVIRAL THERAPY- (02/23/17)
TAF IMPLANT - CROI:
In Vitro -In Vivo Evaluation of a Biodegradable Implant Containing TAF for HIV PrEP
Albuvertide is long acting T20 -Presented at Glasgow2016 : Efficacy and safety of long acting HIV fusion inhibitor albuvirtide in antiretroviral-experienced adults with HIV-1: interim 48 week results from the randomized, controlled, phase 3, non-inferiority TALENT study
http://www.natap.org/2016/GLASGOW/GLASGOW_38.htm

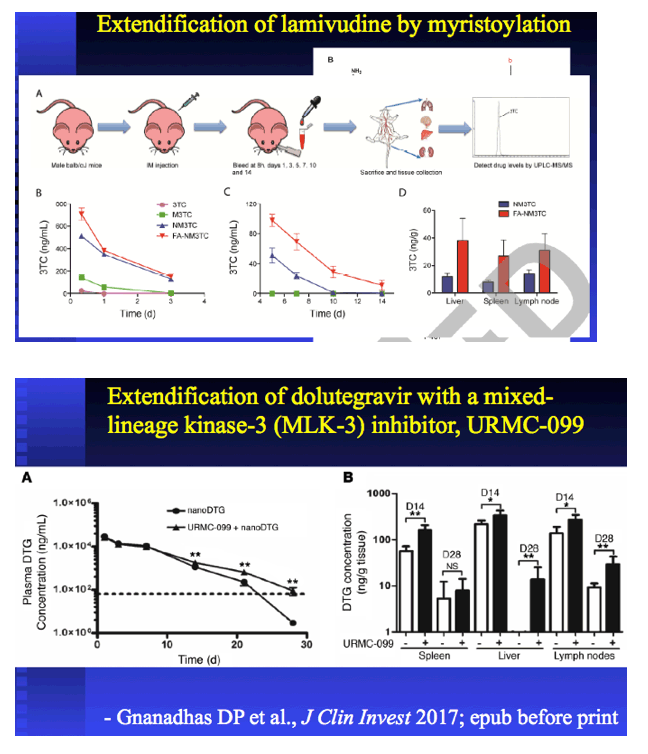
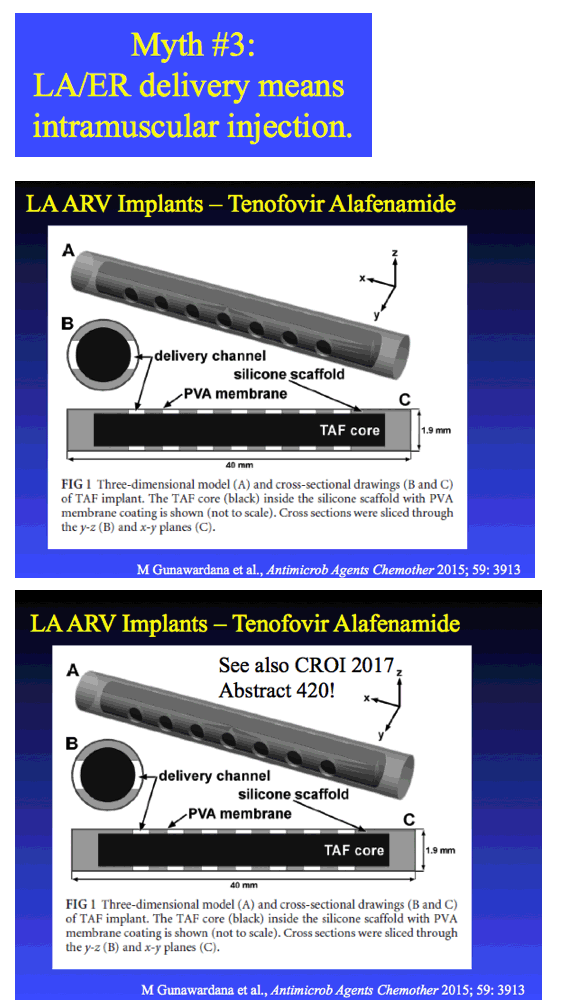
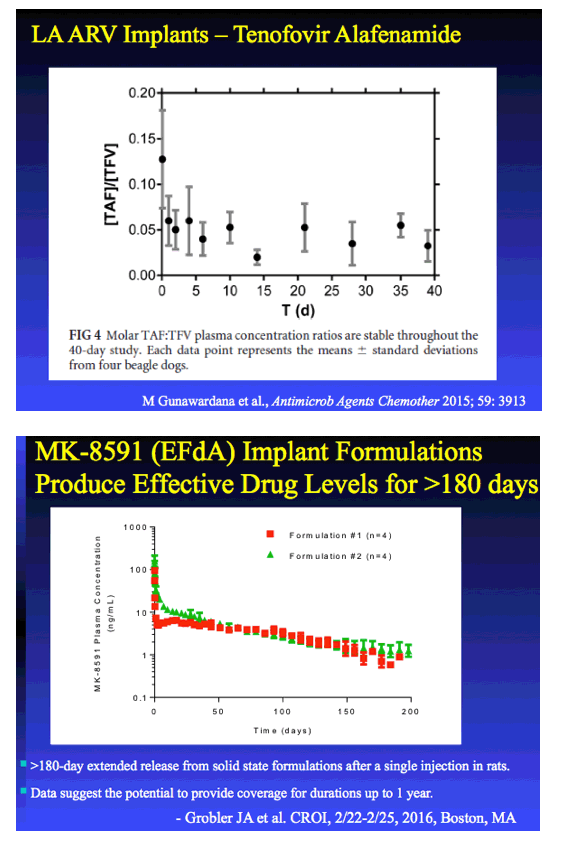
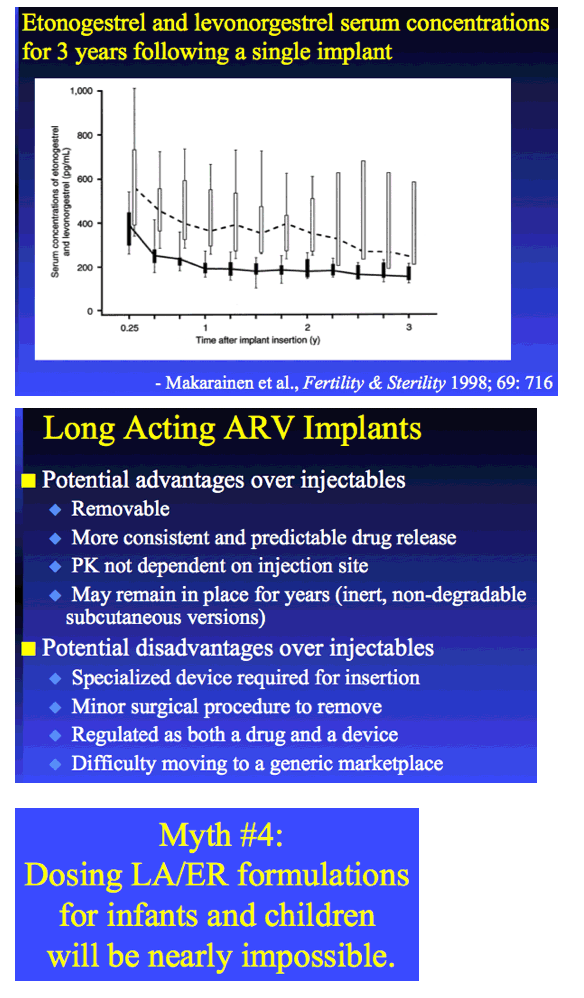
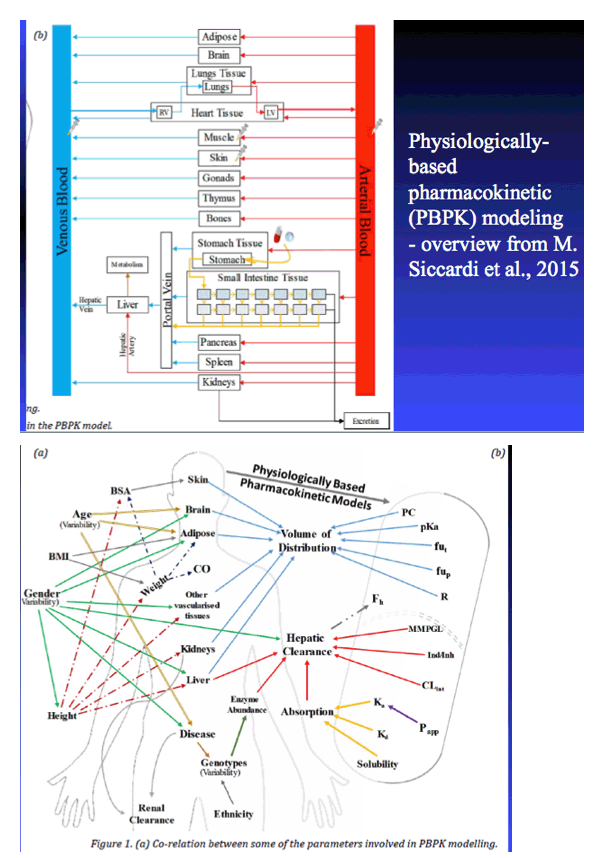
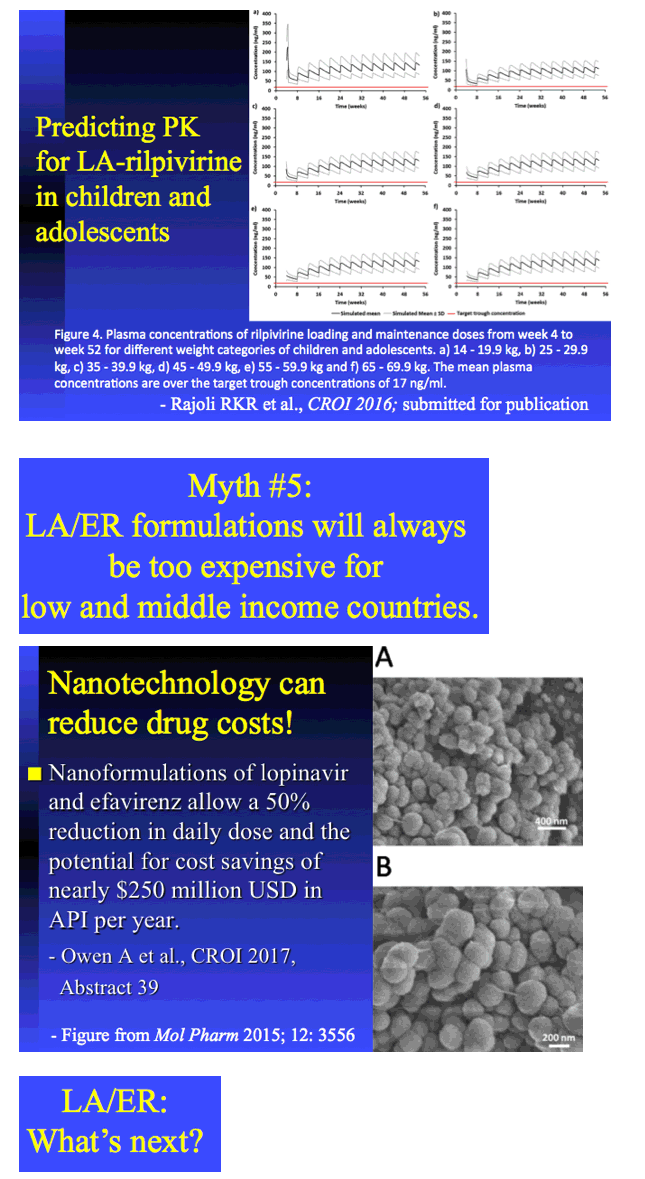

|
|
| |
| |
|
|
|