| |
ViiV Healthcare shares data from its innovative portfolio with HIV community at IAS 2017
|
| |
| |
PRESS RELEASE
22 abstracts related to HIV treatment and prevention to be presented at major international HIV conference
London UK, 20 July, 2017 ‑ ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer Inc. and Shionogi Limited as shareholders, will be presenting 22 abstracts at the annual conference of the International AIDS Society (IAS) 23-26 July, 2017, Paris, France.
John C. Pottage Jr, Chief Scientific and Medical Officer at ViiV Healthcare, commented: "People living with HIV, and their healthcare providers, continue to look for improvements in areas related to their long-term care, such as tolerability, safety, dosing schedules, drug interactions and convenience. The data we're sharing at IAS this year demonstrate our R&D focus on patient-centred care, with a clear drive to find new treatment regimens, new ways to deliver HIV treatments and entirely new molecules to treat and prevent HIV."
While significant progress has been made in tackling the global HIV epidemic, it remains a substantial public health challenge, as 2.1 million people were newly infected in 2014 and more than 36 million people live with HIV worldwide.[i] Data to be presented at IAS support ViiV Healthcare's commitment to delivering advances in treatment and prevention options for people living with HIV (PLHIV). Key data highlights include:
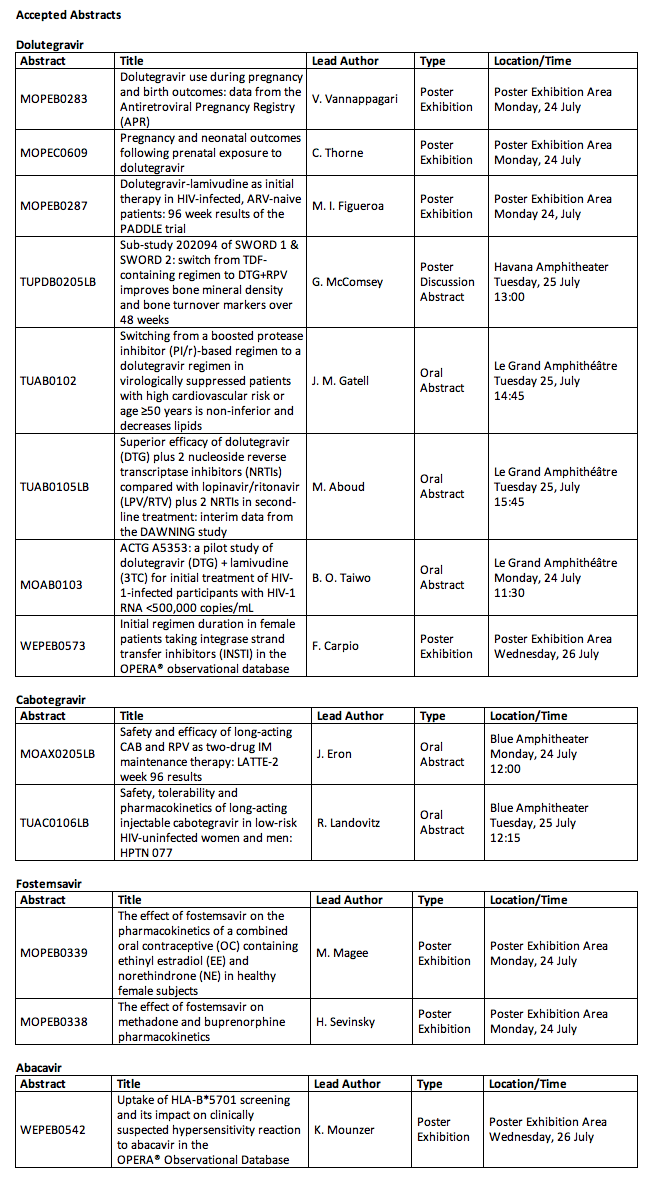
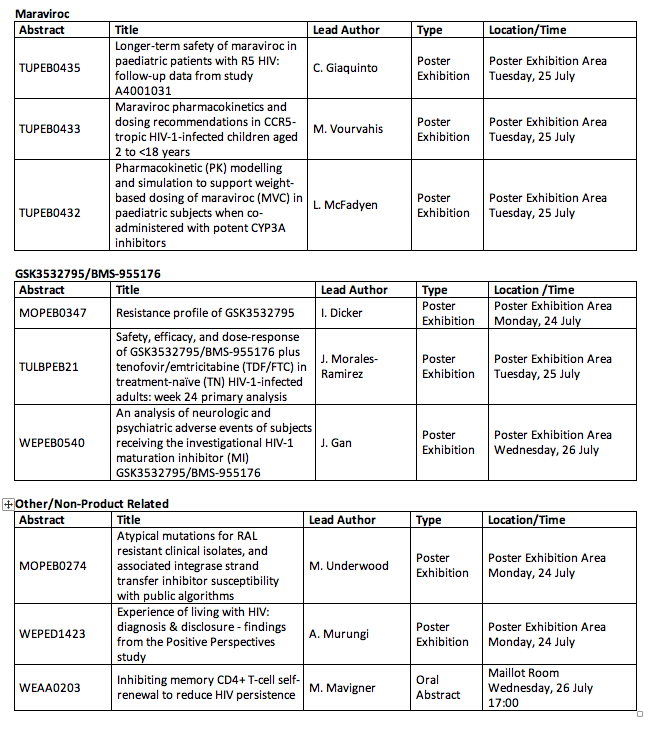
⋅Phase III data comparing a dolutegravir-based regimen against lopinavir/ritonavir-based regimens in patients failing first-line therapy in resource-limited settings (DAWNING)
⋅A sub-study of SWORD 1 & 2: effect of switching from TDF-based regimens to dolutegravir and rilpivirine on bone mineral density and bone turnover markers
⋅Longer-term (96-week) safety and efficacy data from a phase II study of a long-acting, two-drug regimen including cabotegravir and rilpivirine (LATTE-2)
⋅Safety, efficacy and effects on lipid profiles of switching from a boosted protease-inhibitor-based regimen to dolutegravir-based regimen in HIV patients with high CV risk (NEAT 022)
⋅Phase II data from a safety and acceptability study of long-acting, injectable, cabotegravir in HIV-uninfected men and women (HPTN 077)
⋅Phase II safety and efficacy data for a two-drug regimen of dolutegravir and lamivudine in treatment-naïve patients in high- and low-baseline viral load (ACTG 5353)
⋅Results from an international survey of PLHIV to explore the impact of living with an HIV diagnosis on quality of life, outlook and aspirations
These data cover a wide range of important areas in HIV research, such as:
⋅Safety and efficacy in diverse populations including treatment-naïve, treatment-experienced individuals, paediatric patients and women living with HIV who are also pregnant.
⋅Quantitative and qualitative research into the lives of PLHIV, and their needs and expectations.
The breadth of this research is a result of ViiV Healthcare's holistic approach to innovation and dedication to advancing options to help lessen the lifetime burden of HIV therapy on the lives of PLHIV and to reduce the chance of infection altogether.
About ViiV Healthcare
ViiV Healthcare is a global specialist HIV company established in November 2009 by GlaxoSmithKline (LSE: GSK) and Pfizer (NYSE: PFE) dedicated to delivering advances in treatment and care for people living with HIV and for people who are at risk of becoming infected with HIV. Shionogi joined in October 2012. The company's aim is to take a deeper and broader interest in HIV/AIDS than any company has done before and take a new approach to deliver effective and innovative medicines for HIV treatment and prevention, as well as support communities affected by HIV. For more information on the company, its management, portfolio, pipeline, and commitment, please visit www.viivhealthcare.com
About GSK
GSK - one of the world's leading research-based pharmaceutical and healthcare companies - is committed to improving the quality of human life by enabling people to do more, feel better and live longer. For further information please visit www.gsk.com.
[i] UNAIDS. Fact Sheet. Available at: http://www.unaids.org/en/resources/fact-sheet Last accessed July 2017
GSK monitors email communications sent to and from GSK in order to protect GSK, our employees, customers, suppliers and business partners, from cyber threats and loss of GSK Information. GSK monitoring is conducted with appropriate confidentiality controls and in accordance with local laws and after appropriate consultation.
|
|
| |
| |
|
|
|