| |
Neuropsychiatric events and dolutegravir in HIV patients: a worldwide issue involving a class effect
|
| |
| |
Download the PDF
Download the PDF
Kheloufi, Farida,b; Boucherie, Quentinb; Blin, Olivierb; Micallef, Joellea,b
AIDS July 31 2017
We read with interest the work of Menard et al.[1] that brings additional knowledge on the neuropsychiatric tolerance of dolutegravir. They also provide pharmacokinetic data which is very welcomed as published works concerning the potential association between neuropsychiatric adverse drug reactions (ADRs) and dolutegravir concentrations are scarce. Their data are in line with other studies that recently reported neuropsychiatric adverse effects in patients receiving dolutegravir-containing regimen in other countries in Europe [2-4].
However, neuropsychiatric adverse effects in patients treated with integrase strand transfer inhibitors (INSTIs) is not an emergent story [5-7]. One year after raltegravir (Isentress; MSD, Hoddesdon, UK) was marketed, Harris et al. [7] described an exacerbation of depression symptoms in four patients with psychiatric medical history after starting raltegravir. A few months after dolutegravir was available in France, we reported psychiatric disorders in four patients receiving dolutegravir-containing regimen without any other psychoactive comedication [5]. Respectively, these case series contributed to label psychiatric symptoms such as depression or suicidal ideation in the summary of product characteristics of drugs containing raltegravir (Isentress) or dolutegravir (Tivicay, Triumeq; Viiv Healthcare, Brentford, UK). In a case/noncase study of drug-induced depression performed in the French Pharmacovigilance Database, the French pharmacovigilance network found a significant association between raltegravir and depression [8]. Although Harris et al.[7] hypothesized a drug-drug interaction with psychotropic drugs; we suggested a class effect of INSTIs.
In the continuum of our case reports and other published works about neuropsychiatric tolerance of INSTI, we recently reviewed ADRs belonging to the psychiatric disorders system organ class (SOC) of the Medical dictionary for regulatory activities (MedDRA) classification involving an INSTI and registered in the World Health Organization (WHO) worldwide pharmacovigilance database Vigibase between 2007 and April 2017 [9,10]. VigiBase is the international pharmacovigilance database developed and maintained by Upsalla monitoring centre on behalf of WHO in which information is recorded in a structured form to allow analysis of the data. It holds over 15 million anonymized reports of suspected adverse effects of medicines suffered by patients (as of April 2017). This database is at the heart of a global pharmacovigilance system and its purpose is to provide the evidence from which potential medicine safety hazards (signals) may be detected and communicated. Focusing on high level group terms, sleep disorders, depressed mood disorders, anxiety disorders, suicidal and self-injurious behaviours and mood disorders were among the top five reported terms within the psychiatric disorders system organ class independently of the INSTI (Table 1). When using the standardized MedDRA query (SMQ) 'Depression suicide self-injury', we retrieved a higher amount of ADR with raltegravir and dolutegravir compared with elvitegravir.
We believe that this issue is not only focused on Europe and on dolutegravir. Indeed, the presented data are in line with other recent published data suggesting that dolutegravir might be more prone to induce neuropsychiatric ADR compared to raltegravir or elvitegravir. However, it is likely to be an INSTI class effect rather than a phenomenon specific of dolutegravir.
Dolutegravir consistently penetrate central nervous system but to date and to the best of our knowledge, the physiopathological mechanism involved in the onset of neuropsychiatric ADR has not been described [11]. In-vitro studies and the study of potential concentration-related effects would be welcomed to better understand the mechanisms underlying neuropsychiatric tolerance of INSTI, especially dolutegravir.
We acknowledge that our data is not exhaustive because of underreporting of ADRs in addition to the fact that it does not include all ADRs reported to marketing authorization holder. It does not reflect the exact magnitude of neuropsychiatric ADRs involving INSTI which is probably underestimated. Reporting of ADRs is not only compulsory but also a way to contribute to bring knowledge in terms of drug safety. We strongly encourage physicians to report any of these ADR to their local or national health authorities. This knowledge gathered until now should be taken into account during the clinical development of new INSTI (i.e. cabotegravir and bictegravir) that will be marketed in the future years. Indeed, an association with cabotegravir doses and reported rates of insomnia was described in a randomized phase 2b trial [12].
As mentioned by Menard et al.[1], INSTIs are part of first line treatment in HIV therapy. Both patients and physicians should be aware of this issue because ADRs such as insomnia can impair patients' quality of life and adherence to treatment leading to virological failure. More serious ADRs such as suicidal behaviour or depression can also happen while treated with INSTI and should not be neglected or remain in the only scope of dolutegravir.
---------------------
Neuropsychiatric adverse effects on dolutegravir: an emerging concern in Europe
Menard, Ameliea; Montagnac, Clementinea; Solas, Carolineb; Meddeb, Linea; Dhiver, Catherinea; Tomei, Christellea; Ravaux, Isabellea; Tissot-Dupont, Hervea; Mokhtari, Saadiaa; Colson, Philippea; Stein, Andreasa
AIDS May 17 2017
We read with interest the recent articles by Borghetti et al. [1] and Bonfanti et al.[2] that report and make an echo to recent concerns about the neuropsychiatric safety of dolutegravir in the real life setting. This prompted us to review data on dolutegravir use in our own real-life cohort of 2260 HIV-infected patients with a specific focus on neuropsychiatric adverse events (NP-AEs) leading to dolutegravir discontinuation, and on additional pharmacological data when available. We performed a retrospective analysis in patients who had initiated dolutegravir between 1 January 2014 and 30 November 2016, monitored in our infectious diseases unit in public hospitals of Marseille, Southeastern France. A total of 517 dolutegravir-based antiretroviral therapies were initiated during the observation period and 55 AEs (10.6%) led to their discontinuation, with 28 (51%) of these adverse events being NP-AEs. Patients' characteristics and reasons for discontinuation are shown in Table 1. In almost all patients, symptoms occurred during the few months after dolutegravir initiation (median, 4 months), were not life-threatening, did not lead to hospitalization and disappeared quickly after dolutegravir discontinuation (median, 1 month). At initiation of dolutegravir, 94.5% of the patients were pretreated but 52% (29/55) were naïve of integrase strand transfer inhibitor. None of the reported deaths was considered to be drug related. Irritability and sleep disturbances were the most frequently observed NP-AEs. Pharmacological data were available for 12/55 patients with adverse events, including nine with NP-AEs. Median (range) dolutegravir trough concentration (Ctrough) was 1719 ng/ml (811-3730 ng/ml) for those on 50 mg once a day and a Ctrough of 5133 ng/ml was found for the only patient on dolutegravir 50 mg bid.
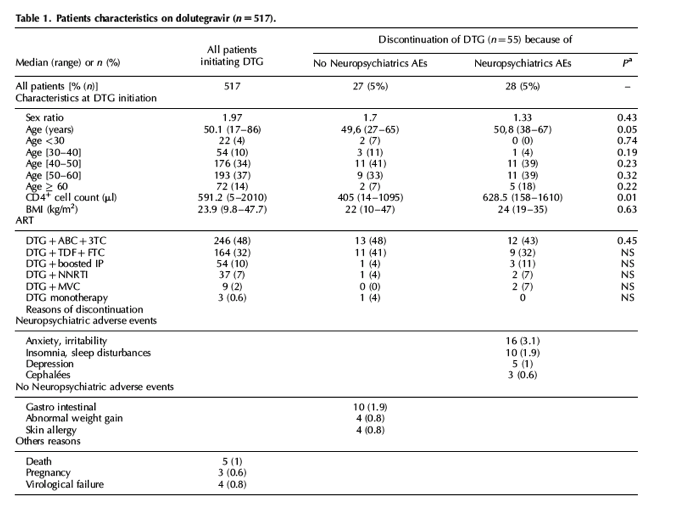
Our rate of dolutegravir discontinuation for NP-AEs (5.4%) is concordant with those reported in most previous real-life studies, which ranged between 3.4 and 6% [1,3,4]. In contrast, this rate was lower in the CISAI real-life study (1%) [2] and in clinical trials [5]. In addition, we did not observe in our larger cohort the association previously described between abacavir administration and NP-AEs [1,3,4]. In multivariate analysis, we found that women with NP-AEs were significantly older (median age, 51 years; range, 44-66) than women who discontinued dolutegravir because of non-NP-AEs (P = 0.03). In previous reports, age more than 60 years [2,3] and female sex [3] were significantly associated with NP-AEs occurrence. Noteworthy, in our study, among male patients who discontinued dolutegravir because of adverse events, BMI was significantly higher in those with NP-AEs than non-NP-AEs (24 versus 20; P = 0.03). Hoffmann et al.[3] suggested an interest in studying the association between BMI and NP-AEs on dolutegravir but to our knowledge we report here for the first time this association. Regarding pharmacological data, dolutegravir Ctrough was supratherapeutic and greater than the median value of 1340 ng/ml found by Yagura et al. as significantly higher in patients with than without NP-AEs (HIV Glasgow 2016; unpublished abstract), which suggested a relationship between dolutegravir exposure and toxicity.
Taken together, previous findings show that NP-AEs emerge as a cause of dolutegravir discontinuation in real-life HIV cohorts in Europe. Other cohort studies at the national and international scale are needed to precise their incidence and putative mechanisms. This is particularly warranted as guidelines have moved to integrase strand transfer inhibitor-based combined antiretroviral therapies for initial treatments.
|
|
| |
| |
|
|
|