| |
Cure Research - Strategies to target HIV-1 in the central nervous system
| | | | |
Download the PDF here
Download the PDF here
Gray, Lachlan R.; Brew, Bruce J.; Churchill, Melissa J.
Current Opinion in HIV and AIDS July 2016
--------------------------
Increases in activation markers and plasma and CSF viral loads were observed in one animal treated with latency reversing agents, despite ongoing ART.....After 500 days of viral suppression animals were treated with two cycles of latency reversing agents and increases in viral transcripts were examined.....Thus, our data suggest that the presence of virus-producing genomes in the CNS should be a cause of concern during AIDS cure strategies aimed at reactivating latent viral genomes. Increased immune activation in the brain, as we observed, may lead to reactivation of latent reservoirs followed by an exacerbated and harmful inflammatory response even in the presence of ART. As the CNS harbors macrophages with persistent replication-competent virus, monitoring CSF for viral activation or residual viremia should be seriously considered during eradication strategies.....http://www.natap.org/2016/HIV/122116_03.htm
"a further adverse outcome, which could be a catastrophic event, is immune reconstitution inflammatory syndromes occurring in the CNS compartment. This could occur due to cytokine storms caused by immunotherapeutic agents modifying neuroinflammatory responses, or immune activation following viral rebound and blips caused by HDAC inhibitors (and similar agents) or viral rebounds associated with antiretroviral treatment interruptions" pdf attached above
ART Interruption Associated with HIV in the Brain: "Reseeding CNS during ART Interruption Leading to Intrathecal Immune Activation" - "HIV-1 Viral Escape in Cerebrospinal Fluid of Subjects on Suppressive Antiretroviral Treatment" - (09/18/17)
Cure Studies/ART Interruption & Brain Affects, Vorinostat - (10/13/17)
-----------------------------------
Purpose of review To review current knowledge of viral reservoirs in the central nervous system (CNS) and identify the CNS-specific barriers and strategies to cure human immunodeficiency virus type 1 (HIV-1) within the brain.
Recent findings The cumulative data of HIV-1 infection of the CNS support the ability of the CNS to act as a viral reservoir for HIV-1. The HIV-1 viral strains found in the CNS are distinct to those found in other parts of the body. These differences have been well documented for env and also extend to the viral promoter, the long terminal repeat, and influence the ability of the virus to replicate, establish latency and respond to latency-reversing agents (LRAs). In addition, the bioavailability and activity of LRAs and antiretrovirals within the CNS suggest altered properties compared with the blood, which may influence their effectiveness. Selected LRAs were shown to have reduced effectiveness against CNS-derived viral strains compared with blood-derived strains from the same patients. Finally, altered immune surveillance within the CNS may also interfere with the efficiency of cure strategies within this compartment.
Summary Together, these data suggest that the CNS viral reservoir is unique and presents a distinct set of challenges that need to be overcome to ensure successful viral elimination within this compartment. Future studies will need to develop CNS-active LRAs and biomarkers to enable monitoring and evaluation of treatment outcomes within the CNS during HIV-1 cure clinical trials.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) cure has proved difficult to achieve because of the persistence of latently infected cells in numerous viral reservoirs throughout the body [1,2]. These reservoirs include resting CD4+ T cells in the blood, as well as other cells in the brain, gut-associated lymphoid tissue, bone marrow and genital tract [3]. Combination antiretroviral therapy (cART) alone is unable to cure HIV-1 as these drugs only target selected steps of the viral life cycle exposed during active replication of the virus, but spare other steps, including early transcription immediately after integration. This has led to the development of several distinct HIV-1 cure strategies, including 'shock and kill', 'bind and gag', CCR5 gene therapy and immune suppression. The best studied strategy, 'shock and kill', 'shocks' latently infected cells into active replication resulting in a burst of viral replication, following the use of a latency-reversing agent (LRAs) [4,5]. The infected cell is then recognized by the immune system and eliminated, while ongoing cART blocks any de-novo infection. The 'bind and gag' strategy uses RNA interference to 'bind' viral RNA and 'gag' it from expressing viral proteins, instead targeting it for degradation [6]. CCR5 gene therapy creates a population of HIV-resistant cells, via ex-vivo modification of autologous patient cells or a bone marrow transplant from a CCR5▵32 homozygous donor [7 ,8]. Finally, immune suppression is being trialed to prevent chronic immune activation and reduce the capacity of the viral reservoir to increase in size [9 ,10].
Although there have been numerous clinical trials with LRAs, understandably the major focus has been on evaluating treatment outcomes in the blood reservoir with only limited attention focussed on tissue reservoirs such as the central nervous system (CNS) reservoir. This is predominantly because of the limitations in the ability to detect and monitor reservoirs in the CNS and the lack of biomarkers available to monitor reactivation of virus and effectiveness of treatments. In addition, several recent studies have presented data that highlight the uniqueness of the CNS reservoir and question whether treatment outcomes in the CNS will mirror those seen in the blood. In this review, we will discuss what is known about the nature of the HIV-1 CNS viral reservoir, specifically, those characteristics unique to the CNS, which constitute predictable barriers to HIV-1 cure and identify additional considerations that need to be addressed to promote optimal and safe eradication of HIV-1 from the CNS reservoir.
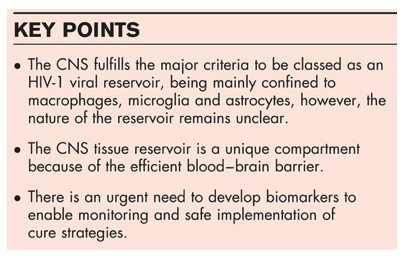
Is the central nervous system a viral reservoir and where is it located?
A previous review by Gray et al. [11 ] addressed whether the CNS is a reservoir for HIV-1, and highlighted that the CNS fulfils most of the criteria required to be classified as a viral reservoir. These criteria include containing an integrated virus, HIV-1 maintained in a latent state, virus exists within long-lived cells and is found at high frequency. It is yet to be shown that the latent virus found in the CNS can be reactivated, and that the virus is replication competent, although several viral proteins (including Tat, Rev and Nef) can be produced in the absence of virion production [12 ]. Integrated virus has been detected in perivascular macrophages, microglia and astrocytes, using laser capture microdissection, coupled with PCR [13,14 ]. However, these analyses have been limited to looking at small regions of the viral genome, due to the fragmented genetic material of formalin-fixed paraffin-embedded tissue samples. A study by Desplats et al. [15 ] identified HIV-1 present in the brains of patients with undetectable viral load in both the blood and cerebrospinal fluid (CSF). They also showed an increase in several epigenetic factors (COUP-TF-interacting protein 2, heterochromatin protein 1, methyl CpG binding protein 2 and histone deacetylase 1), suggesting that these may play a role in maintaining viral latency with the CNS. Furthermore, in our recent publication, we showed that CNS viruses possess unique long terminal repeat (LTR) promoters, with mutations in the Sp motif directly adjacent of the two NF-κB binding sites, which promotes viral latency [16 ]. All of the major HIV-1 target cells within the CNS, that is perivascular macrophages, microglia and astrocytes, are long-lived with half-lives of 3 months, years-lifetime and months-years, respectively [17–20]. These long half-lives allow the virus to persist within CNS cells and enable the maintenance of the CNS viral reservoir. Finally, the frequency of CNS cell infection has been demonstrated in several studies that analyzed macrophages, microglia and astrocytes. Neurocognitively normal patients exhibited 17, 14 and 11% infection of macrophages, microglia and astrocytes, respectively, while impaired individuals exhibited 30, 9 and 19% infection, respectively [14 ,21 ]. In addition, topology studies of infected brain tissue have also revealed that the frequency of HIV-1 infection is highest at or near blood vessels and radiates out with reducing frequency as you move away from the vessel [21 ].
What viral strains constitute the central nervous system viral reservoir?
One important question when discussing HIV-1 cure is whether the viral strains that make up the viral reservoir have unique characteristics, and if so, are these consistent across all viral reservoirs throughout the body. A comprehensive understanding about these viral strains and their characteristics will allow for the design of appropriate cure strategies that specifically target these viral strains. Although full-length viral genomes are yet to be obtained from the CNS reservoir, extensive data exist that provides evidence that the viral strains found with in the CNS are unique. Sequence compartmentalization has been documented between the CNS and non-CNS compartments at the level of HIV-1 Env, Nef and LTR [22 ,23,24]. Recently, it has been demonstrated that CNS-derived LTR sequences contain mutations in a motif normally involved in transcriptional latency, which appear to render the virus more quiescent and may condition the virus into taking on a latent phenotype [16 ]. These mutations were absent from non-CNS-derived LTR sequences from the same patients. It has also been demonstrated that CNS-derived viral strains have a decreased dependence on CD4 for entry and near exclusive use of the CCR5 co-receptor [24–26].
How do we target central nervous system reservoirs?
Assuming the existence of a unique viral reservoir in the CNS, the CNS then poses a number of significant challenges with regard to 'cure' strategies. First, how can the CNS reservoir be efficiently targeted during eradication strategies? Do the drugs used for activation of HIV-1 in the non-CNS compartment adequately penetrate the blood–brain barrier and are they efficient in activating CNS virus? Assuming HIV cure drugs do adequately penetrate the blood–brain barrier and activate CNS virus, how will this virus then be eliminated? The CNS is an immune-privileged compartment and not all antiretrovirals (ARVs) have efficient penetration and effectiveness within the CNS. Protease inhibitors are particularly poor ARVs when it comes to treating CNS infection [27]. Furthermore, it has been demonstrated that even if ARVs have favorable CNS bioavailability, specific ARVs (lamivudine, stavudine and zidovudine) have decreased activity in CNS-derived cells [28 ,29 ]. ARVs have been categorized according to a CNS penetration-effectiveness (CPE) scoring system which is a scale from 1 to 4, with 4 being the most favorable [29 ]. Neurologically active cART refers to cART regimens that have a minimum combined CPE score of 8 or more [30]. Although this scoring system has been criticized, nonetheless, it is a relatively useful tool with some validity for the concept of CNS ARV efficacy [31].Therefore, any cure strategy must ensure that the ongoing cART regimen has good CNS efficacy, likely with a CPE score of at least 8, preferably higher, to ensure the complete blocking of de-novo infection. The problem of poor drug penetration is not restricted to ARVs, LRAs have similar problems, with some such as romidepsin being actively excluded from the CNS [32]. Very recent work has also demonstrated that brain and blood viruses respond very differently to LRAs, with brain viruses being less responsive [16 ]. Finally, while effective delivery or ARVs and LRAs will be important, we must also ensure the immune system present will be sufficiently capable of clearing any cells that harbor reactivated virus. The CNS contains an altered immune system, compared with the rest of the body, because of its relatively immune-privileged status, which may compromise the 'kill' component of the 'shock and kill' strategy within the CNS. Together, these data suggest that the CNS viral reservoir will need a tailored cure strategy employing ARVs and LRAs with high CNS bioavailability and activity against CNS viral strains and involve the augmentation and boosting of the CNS immune system to aid viral clearance. However, this would have to be carefully modulated to avoid, or at least minimize, CNS immune restoration inflammatory syndrome.
How do we identify patients with a significant central nervous system reservoir?
The identification of noninvasive and highly sensitive biomarkers for use in detecting CNS viral reservoirs is a crucial step for the safe implementation of HIV-1 cure strategies. As mentioned previously, not all patients have evidence of a CNS reservoir and those who do, seem to have it to varying extents. This raises the possibility of a threshold CNS reservoir above which specific CNS-targeted cure therapy is needed and below which such treatment may not be required. Thus, a CNS latency biomarker would ideally be quantitative. However, it will not be enough to identify and quantitate CNS latent infection to predict the likelihood of meningoencephalitis in the context of 'shock and kill' eradication therapy. Latent infection in T cells is associated with replication defective HIV-1 in approximately 38% of cells [33]. Something similar is likely for CNS latent HIV-1. Thus, a biomarker of latency significance is required. Currently, there are no such validated biomarkers. However, BCL11b levels in the CSF do hold promise according to Desplats et al. [15 ]. These data need to be replicated and other potential causes of a raised BCL11b such as neurodegeneration need to be addressed [34]. Other potential biomarkers are predicated on the idea that latent HIV-1 in the CNS is not fully latent; that is, there are 'blips' of full or partial replication with consequent CNS damage which may be subclinical. CSF neopterin is elevated in almost all patients with HIV-associated neurocognitive disorders (HAND) on cART and in some without HAND, possibly reflecting such intermittent replication with consequent macrophage/microglial activation [35 ]. CSF neurofilament light chain levels correlate with HAND, and predict its development [36] and thus may also reflect CNS damage from intermittent replication. MRI brain scan with spectroscopy may also be useful in detecting CNS inflammation and injury as a consequence of intermittent replication [37 ].
Is elimination of HIV-1-infected cells of the central nervous system possible and/or desirable?
Cells infected with HIV-1 in the CNS include perivascular macrophages, microglia and astrocytes [13,21 ,38,39]. Astrocytes have been demonstrated to be infected at significant numbers in asymptomatic patients and perform many essential functions within the CNS including neuronal support [40]. Cells within the CNS have a limited capacity to replenish and elimination of significant numbers of infected cells may result in poor patient outcomes. Other strategies aimed at eliminating virus within infected cells are being developed. Inactivation of the CCR5 gene or direct excision and removal of HIV-1 genetic material from infected cells using zinc finger nucleases or CRISPR/Cas9 approaches are showing promise [7 ,8,41 ,42]. However, how these strategies will be delivered to tissue reservoirs such as the CNS remains unclear.
CONCLUSION
Tissue reservoirs including the CNS remain one of the major barriers to HIV-1 'cure'. Specific challenges to HIV-1 cure imposed by the CNS include: the brain is a relatively immunologically privileged compartment, the brain is refractory to adequate penetration by many drugs used in cART, and CNS resident cells have unique transcriptional regulation and viral entry mechanisms. Further, unlike T cells, cells of the CNS have a limited capacity to replenish and eliminating significant numbers of HIV-1-infected cells from the brain may cause neurological damage and result in poor patient outcomes. There is also a lack of biomarkers available to easily and safely monitor reactivation or elimination of virally infected cells. The complete characterization of the CNS reservoir and the development of adequate biomarkers to quantitate and monitor the CNS reservoir are critical to the efficient and safe eradication of HIV-1.
|
| | | | | | |
|