 |
 |
 |
| |
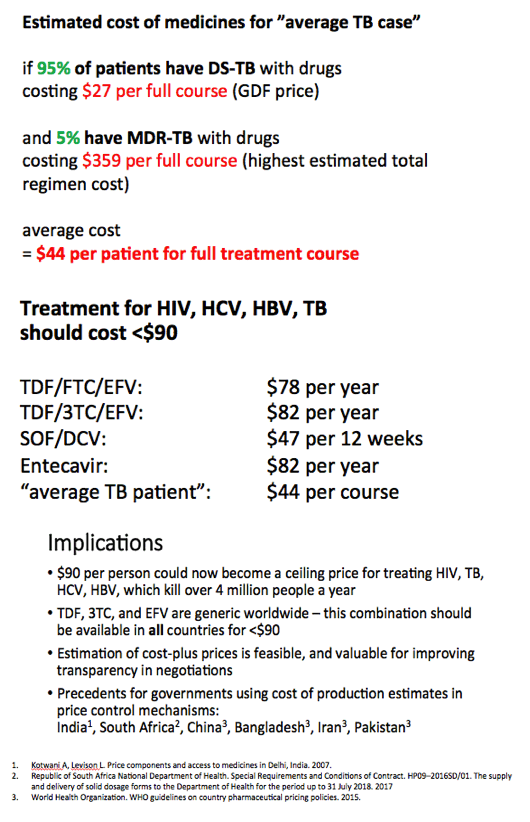
Generic treatments for HIV, HBV, HCV, TB could be mass produced for < $90 per patient
|
| |
| |
Reported by Jules Levin
9th IAS Conference on HIV Science (IAS 2017), July 23-26, 2017, Paris
YouTube webcast: https://www.youtube.com/watch?v=Qo1FtKEkd5Q
A. Hill1, M. Barber2, D. Gotham3, J. Fortunak4, A. Pozniak5
Institutions
1University of Liverpool, Liverpool, United Kingdom, 2London School of Hygiene and Tropical Medicine, London, United Kingdom, 3Imperial College London, London, United Kingdom, 4Howard University, Washington, United States, 5Chelsea & Westminster Hospital, London, United Kingdom
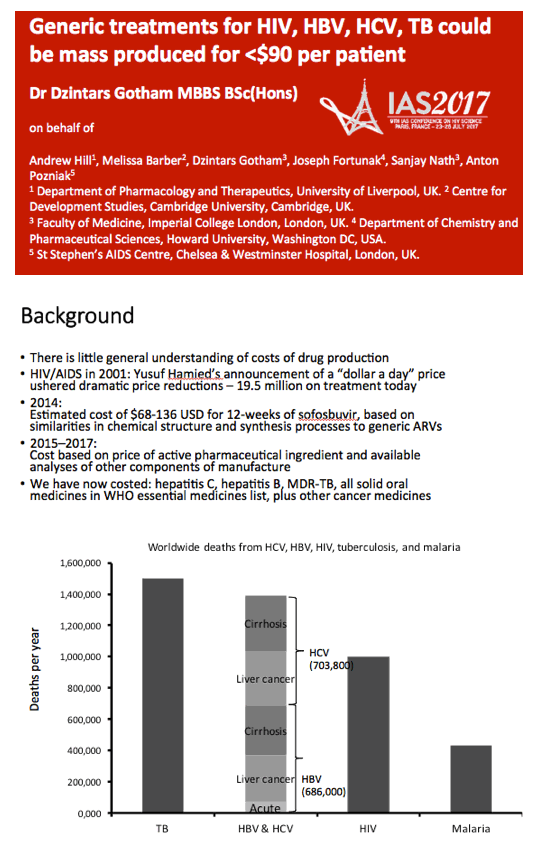
Background: High prices to treat HIV, viral hepatitis and TB can limit treatment access. This analysis aimed to determine prices currently feasible for HIV, HBV, HCV, and first-line (1L) DS-TB treatment, assuming competitive generic manufacture.
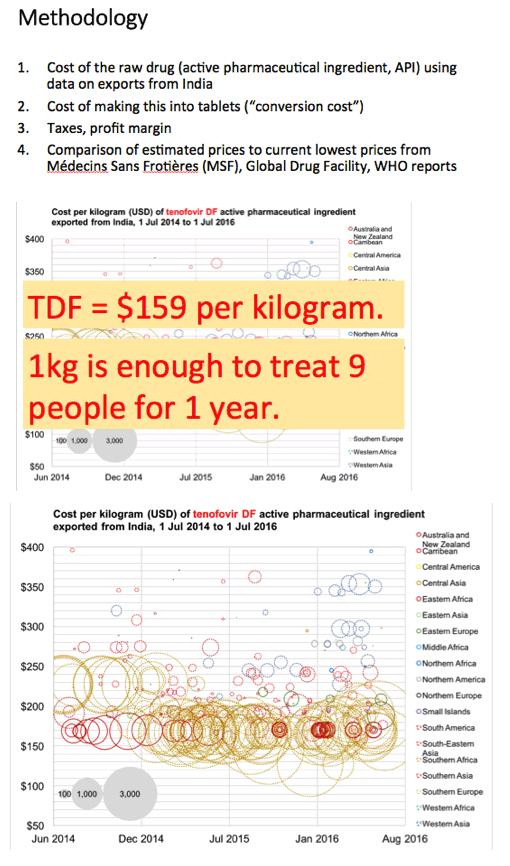
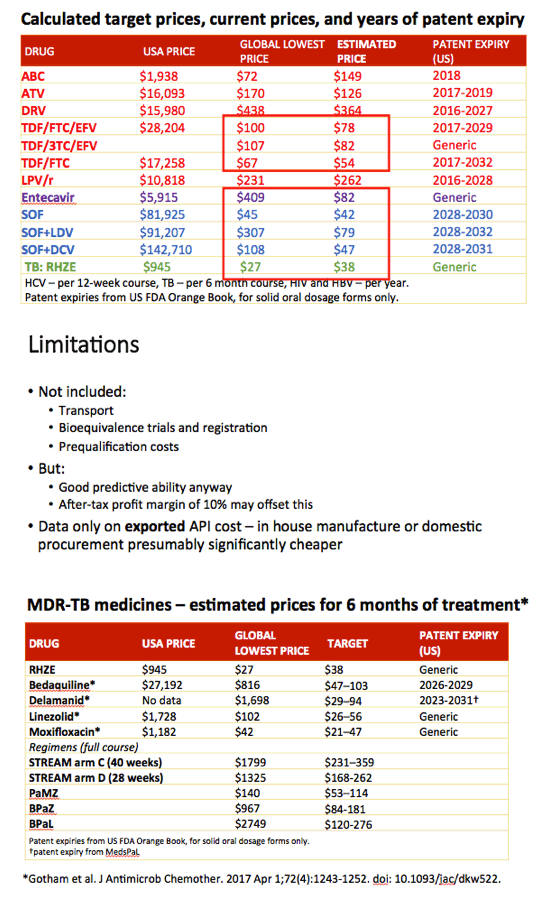
Methods: Data on API exported from India were collected from an online database (www.infodriveindia.com) for July 2014-July 2016. Linear regression was used to plot API cost/kg versus export date, weighted by export volumes: the generated model was used to calculate current average cost/kg of API. Target prices were calculated based on the per-pill cost of API, plus costs of manufacture ($0.01/pill), 10% profit margin, and assumed 27% tax on profit. Current lowest global prices are from public reports and the Global Drugs Fund (TB), US prices from the Centers for Medicare & Medicaid Services. Patent protection expiry dates are from FDA Orange Book and Medicines Patent Pool Patent Status Database.
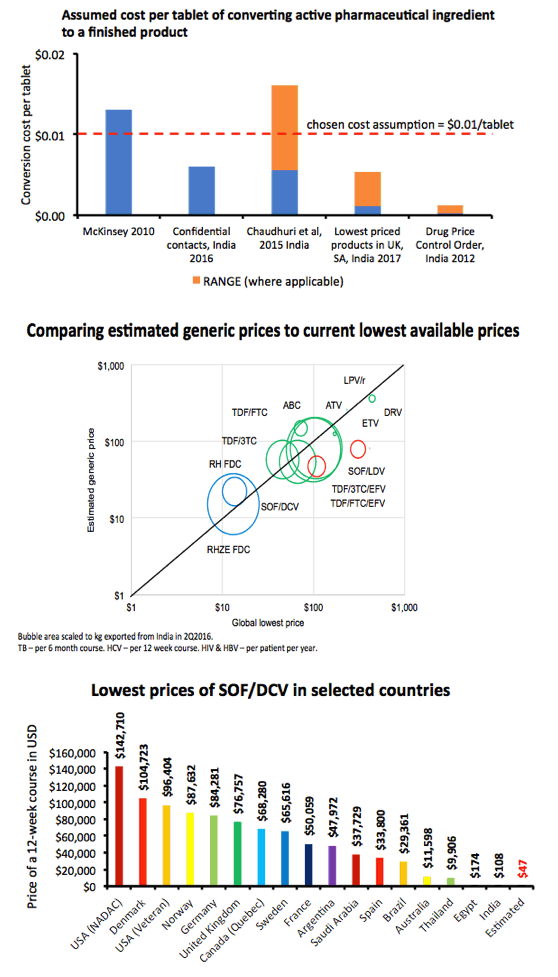
Results: The Table shows current prices of antiretrovirals for HIV, entecavir (ETV) for HBV (per person-year), HCV treatments (per 12-week course) and 1L DS-TB treatment (RHZE, per 6-month course). API costs/kg were $1189 for ATV, $182 for TDF, $241 for 3TC, $109 for EFV, $380,965 for ETV, $1224 for SOF, $4448 for LDV and $852 for DCV. EFV, 3TC, ETV, and RHZE are already generic in USA. The US substance patents on atazanavir expire in 2017, TDF 2018, sofosbuvir 2030, daclatasvir 2031. Sofosbuvir+ledipasvir combination patents expire in 2032.
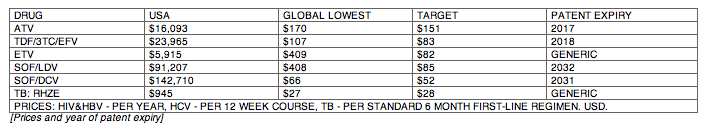
Conclusions: Treatment of HIV, HBV, HCV, and TB could be achieved for < $90 per person globally, if robust generic competition is enabled. In most countries, generic TDF/3TC/EFV, TDF/3TC, ETV or 1L DS-TB treatment could be available for < $90 by early 2018, after patent expiry. Most HCV DAAs will remain on patent for ≥12 more years. Voluntary licensing or other mechanisms will be required to enable access to HCV DAAs at low prices.

|
| |
|
 |
 |
|
|