 |
 |
 |
| |
Dolutegravir / tenofovir / emtricitabine (DTG/TDF/FTC) started in pregnancy is as safe as efavirenz / tenofovir / emtricitabine (EFV/TDF/FTC) in nationwide birth outcomes surveillance in Botswana
|
| |
| |
Dolutegravir Use During Pregnancy and Birth Outcomes: Data From the Antiretroviral Pregnancy Registry (APR) - (08/02/17)
IAS: Superior Efficacy of Dolutegravir (DTG) Plus 2 Nucleoside Reverse Transcriptase Inhibitors (NRTIs) Compared With Lopinavir/Ritonavir (LPV/RTV) Plus 2 NRTIs in Second-Line Treatment: Interim Data From the DAWNING Study - (07/25/17)
Dolutegravir Pharmacokinetics in HIV-Infected Pregnant and Postpartum Women - (03/08/16)
Dolutegravir Effective and Safe in ART-Experienced 6- to 12-Year-Olds - (03/03/16)
Reported by Jules Levin
9th IAS Conference on HIV Science; July 23-26, 2017; Paris, France
R. Zash1,2,3, D. Jacobson4, G. Mayondi3, M. Diseko3, J. Makhema3, M. Mmalane3, T. Gaolathe3, C. Petlo5, L. Holmes6, M. Essex2,3, S. Lockman2,3,7, R. Shapiro2,3
Institutions
1Beth Israel Deaconess Medical Center, Division of Infectious Diseases, Boston, United States, 2Harvard TH Chan School of Public Health, Boston, United States, 3Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana, 4Harvard TH Chan School of Public Health, Center for Biostatistics in AIDS Research, Boston, United States, 5Ministry of Health, Gaborone, Botswana, 6Massachusetts General Hospital for Children, Boston, United States, 7Brigham and Women's Hospital, Boston, United States
Background: Global rollout of DTG-based antiretroviral therapy (ART) has been hampered by lack of safety data in pregnancy.
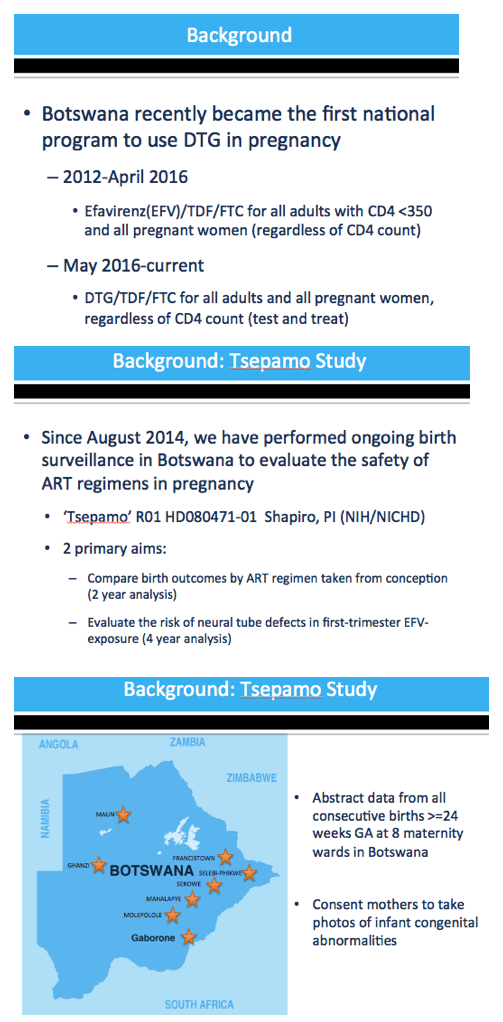
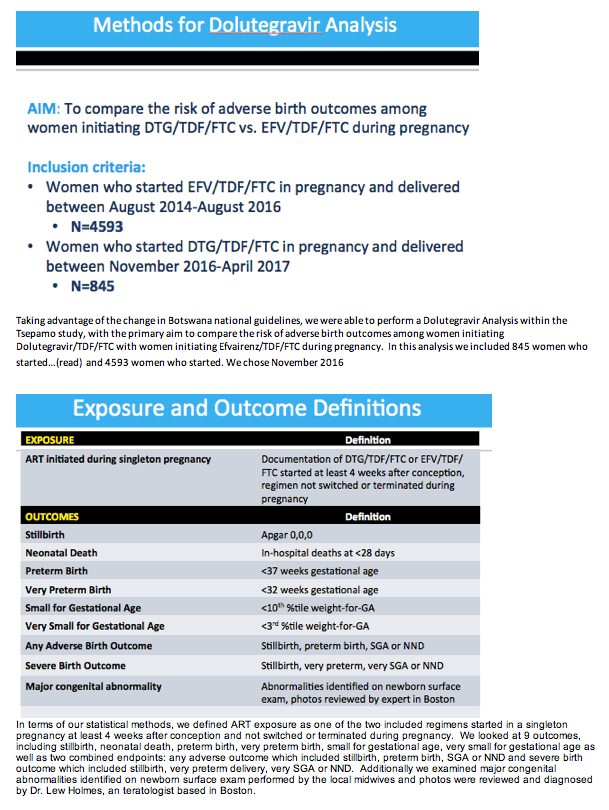
Methods: We captured birth outcomes data at 8 government hospitals throughout Botswana (∼45% of all deliveries) starting August 2014. In 2016, Botswana changed first-line ART from EFV/TDF/FTC to DTG/TDF/FTC, including for pregnant women. This analysis included women initiating either EFV/TDF/FTC (delivered August 2014 to August 2016) or DTG/TDF/FTC (delivered November 2016 to April 2017) during singleton pregnancy. Outcomes included combined endpoints of any adverse outcome (stillbirth, preterm birth [< 37 weeks], small for gestational age (SGA)[< 10th% weight-for-gestational age], or neonatal death [< 28 days]) and severe adverse outcomes (stillbirth, neonatal death, very preterm birth [< 32 weeks] and very SGA [< 3rd% weight-for-gestational age]). We fit log-binomial regression models, controlling for maternal age, gravidity and education, to estimate adjusted risk ratios (aRRs). Congenital abnormalities were detected by maternity nurse surface exam.
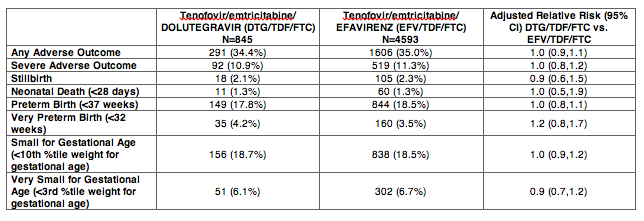
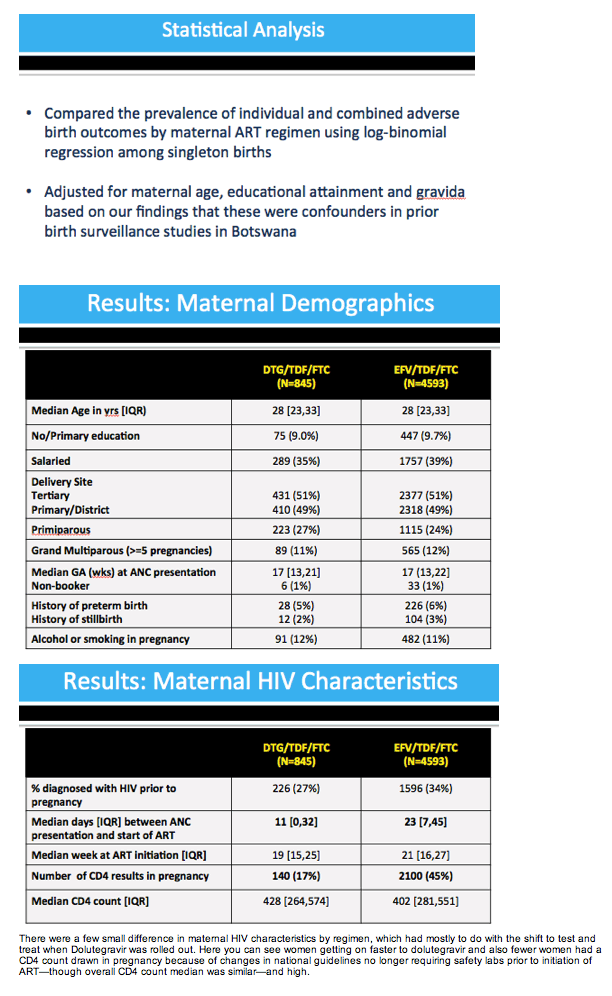
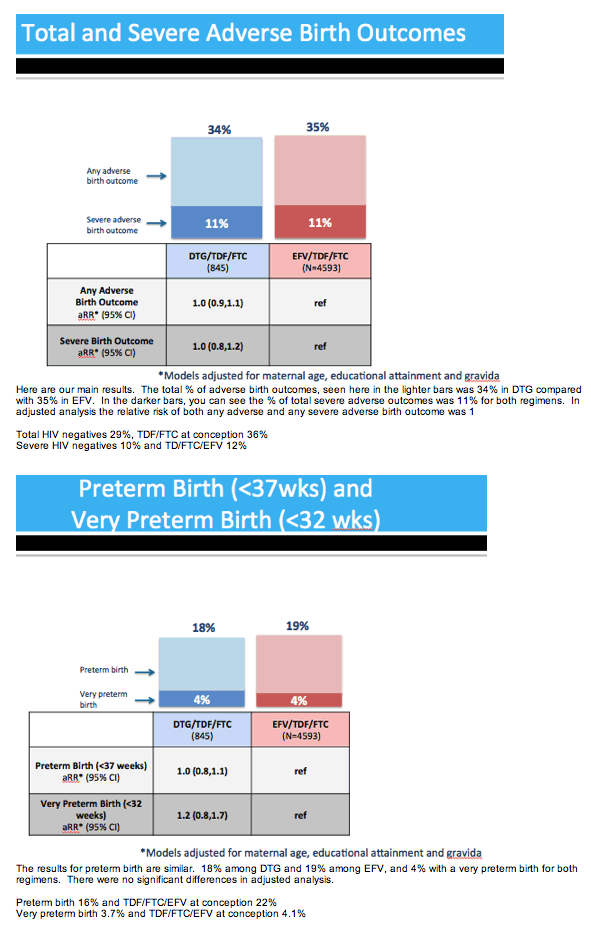
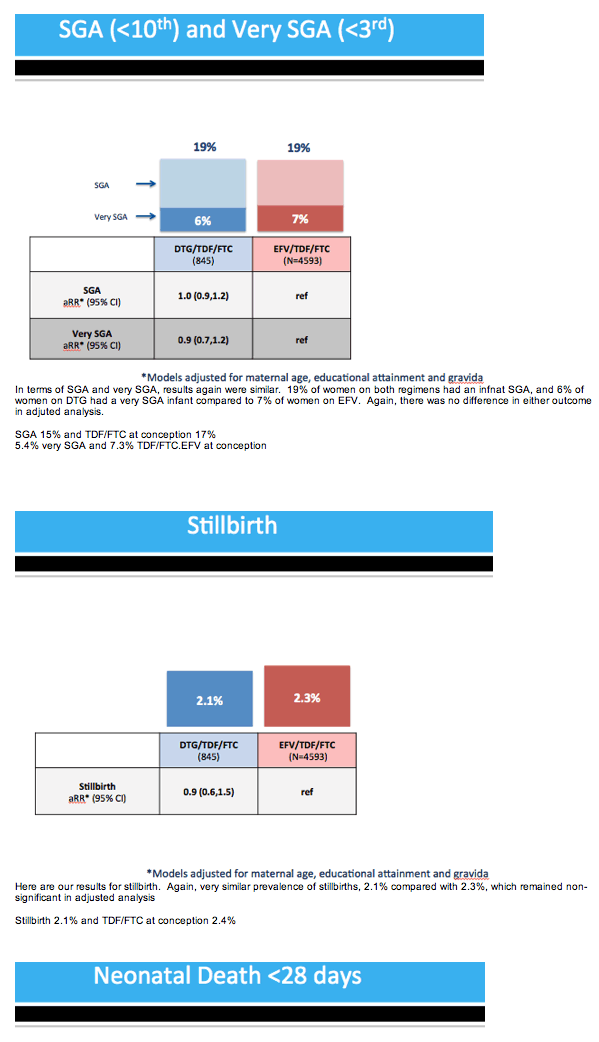
Results: Maternal characteristics were similar for women starting DTG/TDF/FTC (N=845) or EFV/TDF/FTC (N=4593), including age, education, occupation, parity, alcohol/tobacco use, history of adverse birth outcome, delivery site, gestational age at presentation for antenatal care, and CD4 cell count. ART was initiated at median [IQR] 19 [15, 25] weeks gestation for DTG/TDF/FTC and 21 [16, 27] for EFV/TDF/FTC. There were no significant differences in stillbirth, neonatal death, preterm or very preterm birth, SGA or very SGA by regimen (Table 1). Comparisons of any adverse birth outcome (aRR 1.0, 95%CI 0.9,1.1) and severe outcomes (aRR 1.0, 95%CI 0.8,1.2) were similar by regimen. Among 512 first-trimester ART initiations (116 DTG/TDF/FTC, 396 EFV/TDF/FTC), one major congenital abnormality was identified (skeletal dysplasia in an EFV-exposed infant).
Conclusions: Adverse birth outcomes were similar for DTG-based ART and EFV-based ART when started during pregnancy. Further studies are needed to determine the safety of DTG exposure from conception.

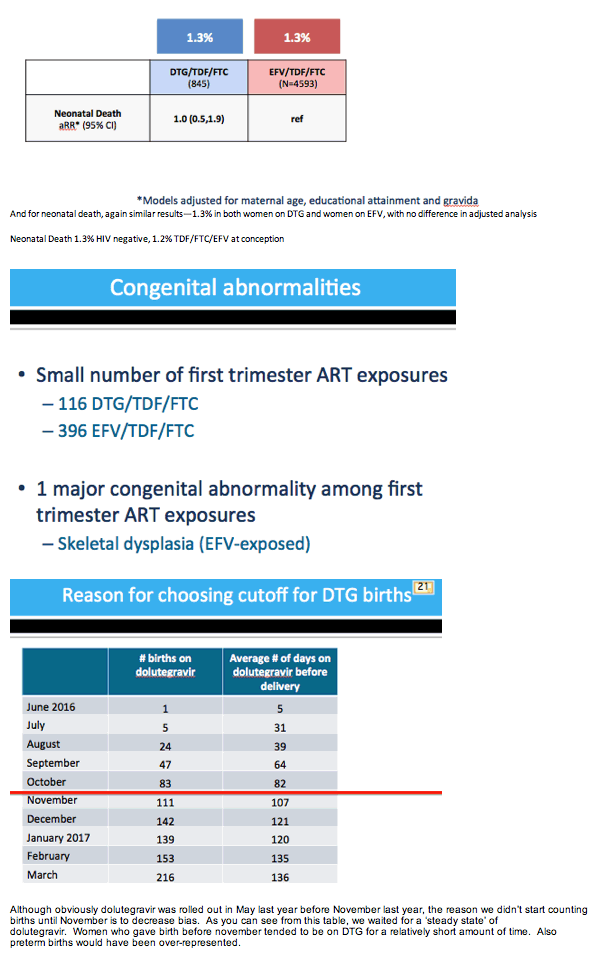

|
| |
|
 |
 |
|
|