 |
 |
 |
| |
Maintenance With Raltegravir/Etravirine Strong at 48 Weeks in Older Adults - Efficacy of a Maintenance Strategy with Raltegravir/Etravirine : the ANRS 163 ETRAL trial
|
| |
| |
9th IAS Conference on HIV Science (IAS 2017), July 23-26, 2017, Paris
Mark Mascolini
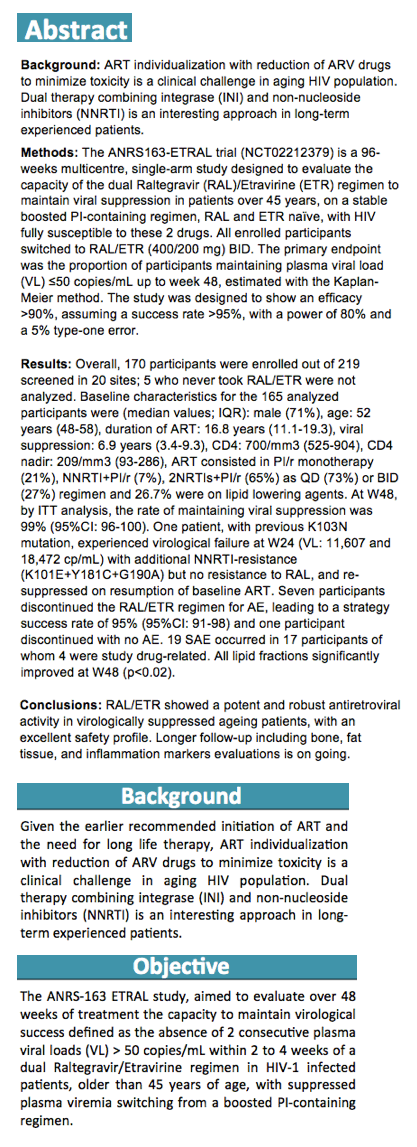
A high proportion of 165 people who switched from a stable boosted protease inhibitor (PI) to raltegravir/etravirine (RAL/ETV) maintained virologic suppression through 48 weeks in a French trial enrolling people 45 or older [1]. Seven participants stopped the regimen because of adverse events, and 1 with a prior nonnucleoside mutation had virologic failure.
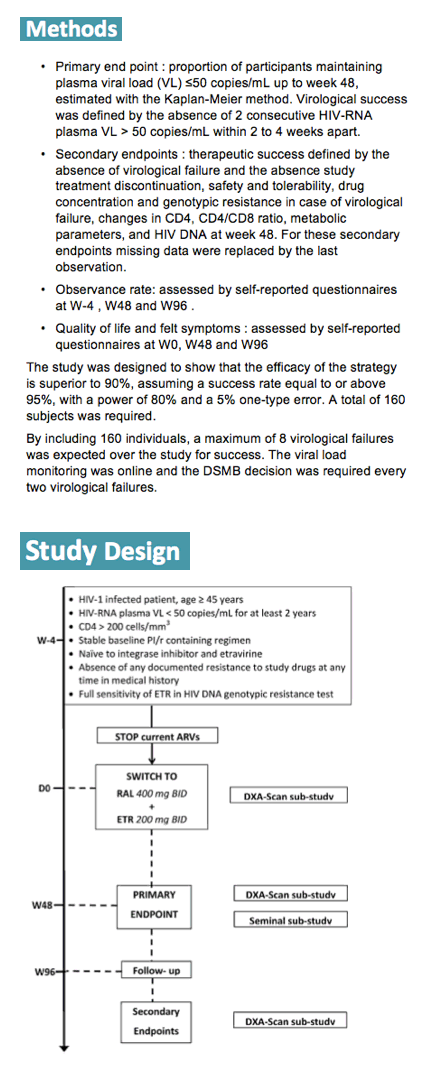
The search is on for a safe two-antiretroviral combination that can maintain HIV control achieved with more complex or toxic regimens. French ANRS investigators aiming to replace a ritonavir-boosted PI regimen in older adults nominated RAL/ETV (an integrase inhibitor and a nonnucleoside) as their candidate maintenance medley. The objective of the ANRS-163 ETRAL trial was to evaluate RAL/ETV after a stable boosted PI for its ability to maintain virologic control [2], defined as absence of two consecutive loads above 50 copies through 48 weeks. Enrolling 160 participants, the ANRS team expected a maximum of 8 virologic failures in 48 weeks.
The investigators recruited people at least 45 years old who maintained a viral load below 50 copies for at least 2 years and took a current boosted PI regimen. No one had taken an integrase inhibitor (such as raltegravir) or etravirine, and no one had resistance to either study drug at any time. All participants traded their PI combination for RAL/ETV at a dose of 400/200 mg twice daily. The primary endpoint was proportion with a viral load below 50 copies through 48 weeks.
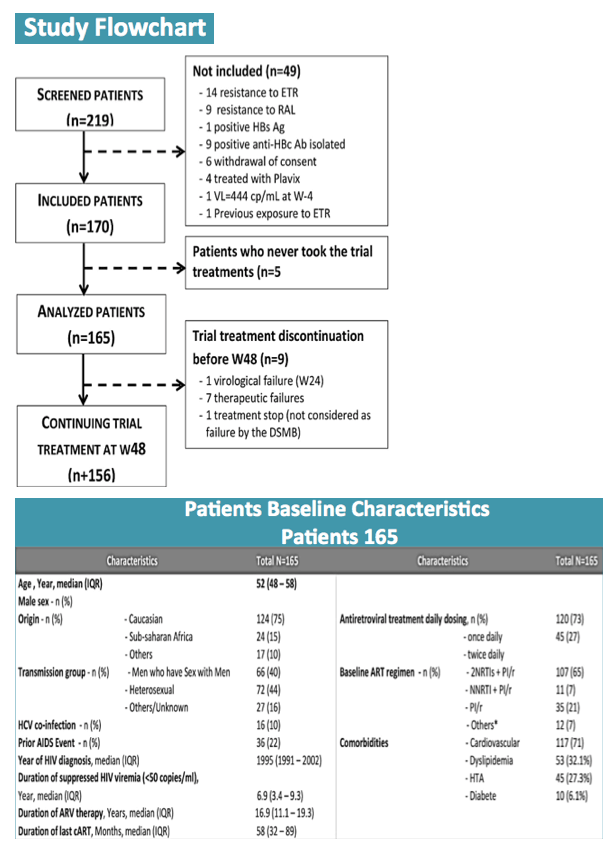
Among 170 participants, 5 never started RAL/ETV and were not included in the analysis. Median age of the 165 participants stood at 52 years (interquartile range 48 to 58), 75% were white, 71% were men, and 40% were men who have sex with men. Enrollees had taken antiretroviral therapy for a median of 16.8 years. Two thirds of participants (65%) were taking a boosted PI plus two nucleosides, 21% were taking boosted-PI monotherapy, 7% were taking a boosted PI plus a nonnucleoside, and the rest were taking other combinations. Three quarters (73%) had a once-daily baseline regimen.
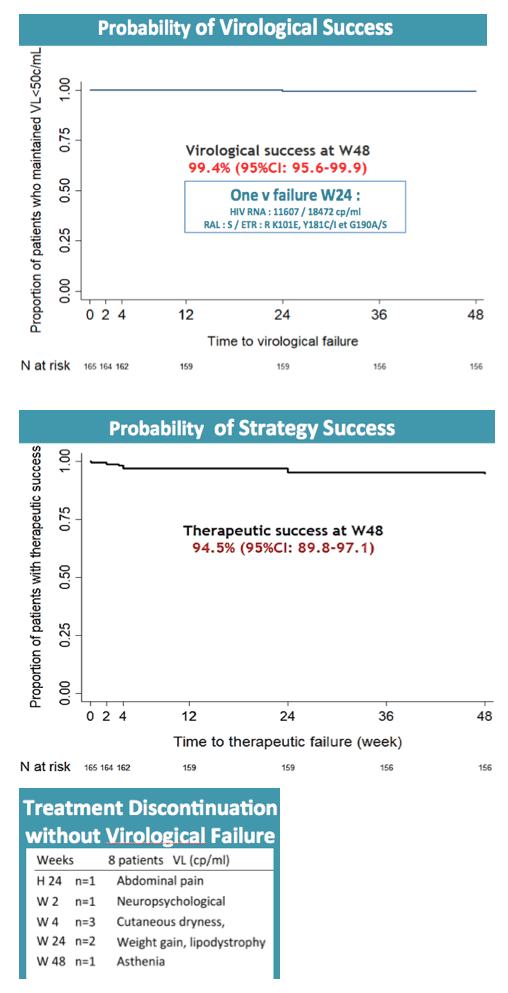
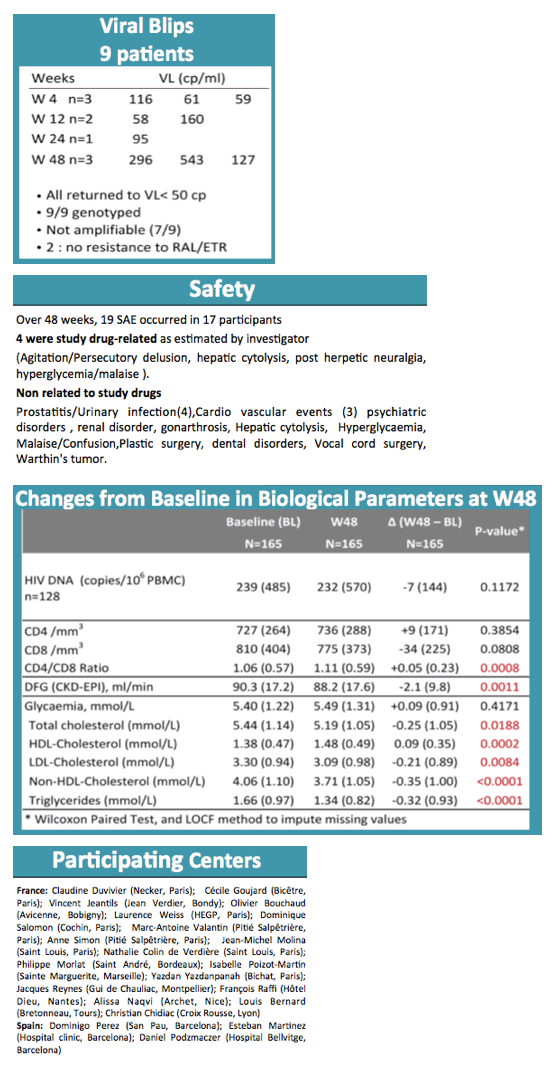
By week 48 only 1 participant had virologic failure, and this person turned out to have a previous nonnucleoside mutation, K103N. Three additional nonnucleoside mutations emerged with this failure. The participant regained viral control after resuming the baseline regimen. Thus the 48-week virologic success rate was 99.4% (95% confidence interval [CI] 95.6 to 99.9). Nine participants (5%) had isolated blips above 50 copies/mL, none of whom had detectable raltegravir or etravirine mutations as a result.
In addition to the 1 virologic failure, there were 7 therapeutic failures due to adverse events to yield a therapeutic success rate of 94.5% (95% CI 89.8 to 97.1). Among 19 serious adverse events, researchers rated 4 related to study drugs, including agitation with a persecutory delusion, hepatic cytolysis, postherpetic neuralgia, and hyperglycemia/malaise.
Baseline parameters that improved significantly through 48 weeks were CD4/CD8 ratio (+0.05), total cholesterol (-0.25 mmol/L), HDL cholesterol (+0.09 mmol/L), LDL cholesterol (-0.21 mmol/L), non-HDL cholesterol (-0.35 mmol/L), and triglycerides (-0.32 mmol/L) (all P < 0.02). Continuing follow-up will include assessments of bone markers, inflammation markers, and fat tissue.
Despite twice-daily dosing with RAL/ETV, the ANRS team calculated an adherence rate above 95%.
References
1. Katlama C, Reynes J, Assoumou L, et al. Raltegravir/etravirine as maintenance strategy in HIV-1-infected virologically suppressed individuals aged over 45 years on prior boosted protease inhibitor containing regimen: results at W48 of the ANRS163-ETRAL study. 9th IAS Conference on HIV Science (IAS 2017), July 23-26, 2017, Paris. Abstract MOPEB0314.
2. ClinicalTrials.gov. Capacity of the dual combination raltegravir/etravirine to maintain virological success in HIV-1 infected patients of at least 45 years of age--ANRS 163 ETRAL. ClinicalTrials.gov identifier NCT02212379.
https://clinicaltrials.gov/ct2/show/NCT02212379
-----------------------------
Efficacy of a Maintenance Strategy with Raltegravir/Etravirine : the ANRS 163 ETRAL trial
C Katlama1, J Reynes2, L Assoumou3, MA Valantin1, L Beniguel3, C Soulie4, O Bouchaud5, F Raffi6, E Martinez7, AL Argoud8,
S Gibowski8, G Peytavin9, AG Marcelin4, D Costagliola3
1APHP hopital Pitie Salpetriere, Sorbonne Universites, INSERM, UPMC Univ Paris 06, Institut Pierre Louis d'epidemiologie et de Sante Publique (IPLESP UMRS 1136), Paris, France ; 2CHU de Montpellier et UMI IRD233-INSERM U1175-Universite de Montpellier ; 3Sorbonne Universites, INSERM, UPMC Univ Paris 06, Institut Pierre Louis d'epidemiologie et de Sante Publique (IPLESP UMRS 1136), Paris, France ; 4Laboratoire de Virologie, Sorbonne Universites, INSERM, UPMC Univ Paris 06, Institut Pierre Louis d'epidemiologie et de Sante Publique (IPLESP UMRS 1136), Paris, France ; 5 APHP hopital Avicenne et Universite Paris 13, IMEA-Fondation Internationale Leon Mba ; 6 University Hospital Hotel Dieu- Nantes, France; 7 Infectious Diseases Unit Hospital Clínic - IDIBAPS University of Barcelona, Barcelona, Spain; 8 ANRS, France Recherche Nord & Sud Sida-HIV Hepatites, Paris, France ; 9 AME, Inserm UMR 1137, Universite Paris 7, UF 301 Laboratoire de Pharmaco-Toxicologie, GH X Bichat-Cl Bernard, Paris, France
|
| |
|
 |
 |
|
|