 |
 |
 |
| |
Evaluation of the risk of birth defects among children exposed to raltegravir in utero in the ANRS-French Perinatal Cohort EPF - Raltegravir Not Tied to Birth Defects in French Study of 479 Births
|
| |
| |
9th IAS Conference on HIV Science (IAS 2017), July 23-26, 2017, Paris
Mark Mascolini
Birth defect rates proved similar in infants exposed to raltegravir in utero and those exposed to
any antiretroviral in the largest such raltegravir study to date [1]. Analysis of 479 births in the ANRS-French Perinatal Cohort EPF found birth defect rates did not differ significantly between starting raltegravir in the first trimester and starting in the second or third trimester.
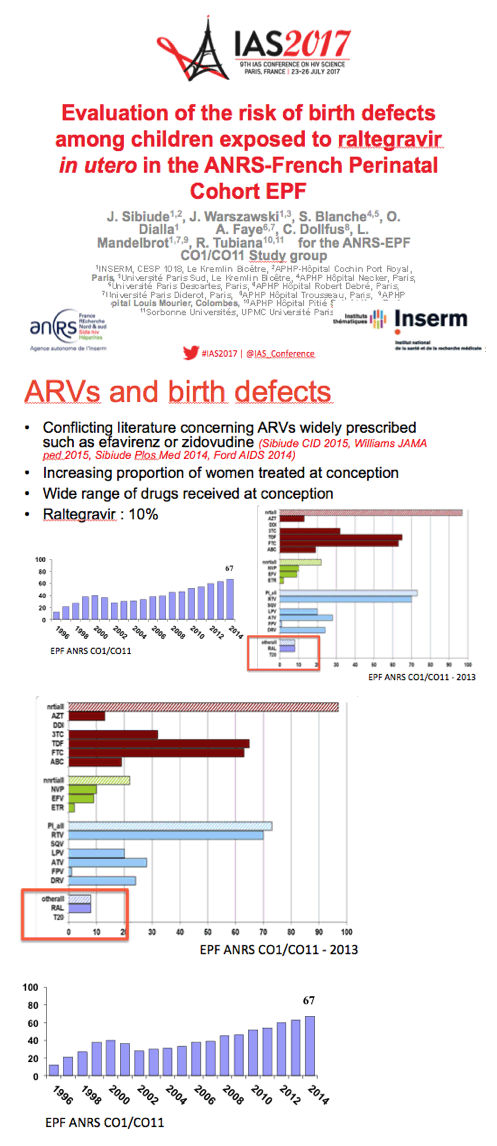
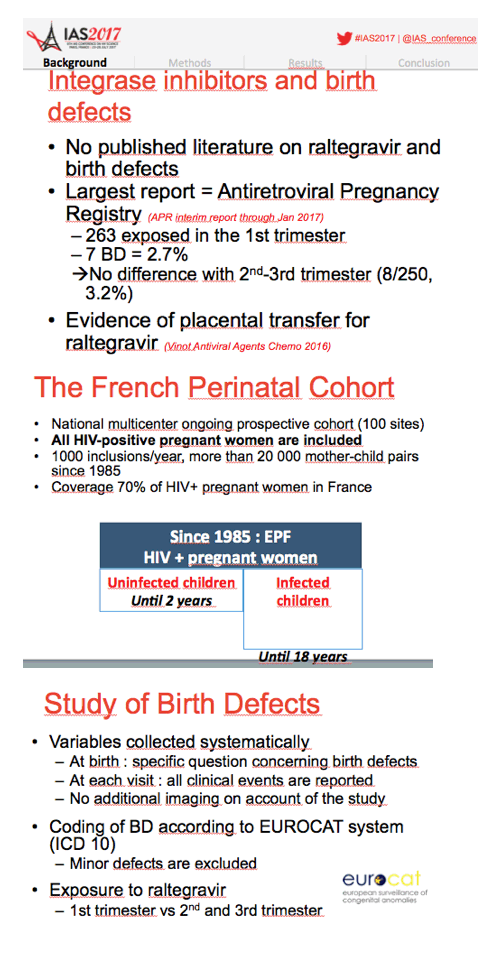
Because raltegravir has seen wide use over recent years but data are scarce on its in utero impact, the ANRS team conducted this analysis of the 100-site national EPF cohort, which prospectively enrolls pregnant women with HIV. Establishing the safety of individual antiretrovirals in pregnancy has gained even more importance since growing proportions of women are taking antiretrovirals when they conceive.
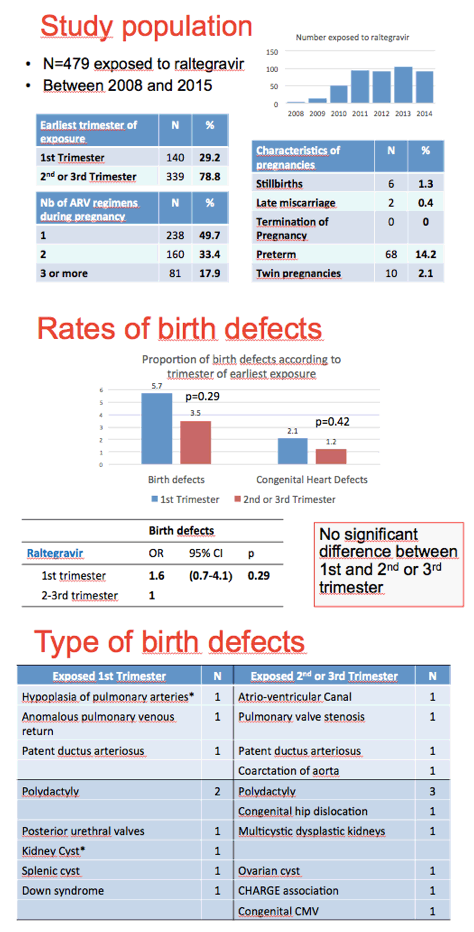
The EPF cohort enrolls about 1000 HIV-positive pregnant women yearly and includes more than 20,000 mother-child pairs since 1985. The researchers identified birth defects according to the EUROCAT system; they excluded minor birth defects. The investigators figured that about 10% of pregnant woman took raltegravir in 2013. This analysis focused on 479 fetuses exposed to raltegravir between 2008 and 2015, including 140 first-trimester exposures and 339 second- or third-trimester exposures.
Six pregnancies (1.3%) ended in stillbirths, 2 (0.4%) in late miscarriages, and 68 (14.2%) in preterm deliveries. The researchers counted 20 birth defects in all 479 births (4.2%, 95% confidence interval [CI] 2.4% to 6.0%) and 20 birth defects in 471 live births (4.2%, 95% CI 2.4% to 6.1%). These rates are similar to the 4.4% rate previously recorded in the EPF cohort for live births exposed to any antiretroviral (95% CI 4.0% to 4.7%).
Birth defect rates were 5.7% with earliest raltegravir exposure in the first trimester and 3.5% with earliest exposure in a later trimester. This difference lacked statistical significance (P = 0.29). Congenital heart defects occurred in 2.1% of first-trimester exposures and 1.2% of later-trimester exposures, also a nonsignificant difference (P = 0.42). Birth defects followed no particular pattern in the first trimester or in the second-and-third trimester.
The ANRS investigators concluded there is "no evidence of [an] association between birth defects and exposure to raltegravir in utero."
Reference
1. Sibiude J, Warszawski J, Blanche S, et al. Evaluation of the risk of birth defects among children exposed to raltegravir in utero in the ANRS-French Perinatal Cohort EPF. 9th IAS Conference on HIV Science (IAS 2017), July 23-26, 2017, Paris. Abstract MOAB0204.
----------------------
Evaluation of the risk of birth defects among children exposed to raltegravir in utero in the ANRS-French Perinatal Cohort EPF
J. Sibiude1,2, J. Warszawski1,3, S. Blanche4,5, O. Dialla1 A. Faye6,7, C. Dollfus8, L. Mandelbrot1,7,9, R. Tubiana10,11 for the ANRS-EPF CO1/CO11 Study group
1INSERM, CESP 1018, Le Kremlin Bicetre, 2APHP-Hopital Cochin Port Royal, Paris, 3Universite Paris Sud, Le Kremlin Bicetre, 4APHP Hopital Necker, Paris, 5Universite Paris Descartes, Paris, 6APHP Hopital Robert Debre, Paris, 7Universite Paris Diderot, Paris, 8APHP Hopital Trousseau, Paris, 9APHP Hopital Louis Mourier, Colombes, 10APHP Hopital Pitie Salpetriere, Paris, 11Sorbonne Universites, UPMC Universite Paris


|
| |
|
 |
 |
|
|